1.3 Chemical Bonds and Molecules
Learning Objectives
By the end of this section, you will be able to:
- Descibe important chemical bondings include ionic and covalent bonds
- Explain how the hydrogen bonds of water are responsible for its remarkable properties
A molecule is matter that contains more than one atom. Molecules that contain more than one element in a fixed ratio are called compounds. Atoms in molecules and compounds are held together by chemical bonds. Chemical bonds are forces between atoms that hold them together.
The outermost electron shell of an atom is called the valence shell and the electrons within that shell are called valence electrons. They are the highest energy electrons in an atom. The valence electrons may be transferred or shared with other atoms. The valence number of an element is the number of electrons required to fill the valence shell.
Reactive elements are elements with incomplete valence shells. For example, hydrogen (H) has an atomic number of 1. The valence shell is the first shell and that shell contains only 1 electron. The first shell can accommodate two electrons, so the valence shell is incomplete and H is reactive. Generally, these atoms will share or transfer valence electrons easily. In contrast, inert elements are elements with full or complete valence shells. These atoms do not share or transfer electrons easily. For example, helium (He) has an atomic number of two. The valence shell of He is the first shell and it contains the maximum two electrons. Therefore, He is inert.
Ionic bonds
Reactive atoms that are close to filling their valence shells will pull on the valence electrons of nearby atoms with force. This property is called electronegativity. The closer an atom is to filling its valence shell, the more electronegative that atom is. For example, chlorine (Cl) has an atomic number of 17. The valence shell of Cl contains seven out of a total possible eight electrons. Cl requires only one electron to fill its valence shell and it is very electronegative compared to sodium (Na), for example. Na has an atomic number of 11 and its valence shell contains one out of a possible eight electrons. Therefore, Cl will pull on the single valence electron of Na with force. Na will transfer its valence electron to Cl, resulting in two ions: Na+ because Na has transferred a valence electron to Cl and Cl– because it has gained an electron. The two oppositely charged ions are attracted to one another. The resulting electrostatic attraction between Na+ and Cl– is called an ionic bond and the resulting compound is sodium chloride (NaCl).
IMPORTANT
Covalent bonds
Atoms with similar electronegativities will not transfer electrons to form an ionic bond between one another. Instead, they may share electrons to form a covalent bond. For example, carbon (C) has an atomic number of 6 and hydrogen (H) has an atomic number of 1. The valence shell of H is the second shell and it is half-full. The valence shell of H is the first shell and it is also half-full. Therefore, these atoms have similar electronegativities. When they come into proximity of one another, the valence electrons of C will complete the valence of H atoms and vice versa. Because these atoms pull on the shared electrons with equal force, the bonds between C and H are nonpolar covalent bonds. The electrons are shared equally between the bonding partners.
In contrast, oxygen (O) has an atomic number of 8 and its valence shell is the second shell. O is much closer to filling its valence shell than H; therefore, O is more electronegative than H. O and H can react to form water (H2O). In water, O and H will share their valence electrons to fill each other’s valence shells. However, because O pulls on electrons with more force, the shared electrons tend to reside with O more often than with either H atom. That means that O carries a partial negative charge (δ-) and C carries a partial positive charge (δ+), resulting in a polar covalent bond between the atoms. Covalent bonds are relatively strong bonds as compared to electrostatic interactions such as ionic or hydrogen bonds.
IMPORTANT: Molecules and compounds are not the same thing!
The atoms in molecules are joined by covalent bonds. Compounds contain two different atoms joined by either covalent or ionic bonds. Molecular oxygen (O2) is a molecule containing two of the same atoms joined by nonpolar covalent bonds. However, O2 is not a compound because it contains only one type of atom. Water (H2O) is both a molecule and a compound because the atoms in water are joined by covalent bonds and it contains two different atoms: O and H.
Chemistry in the clinic:
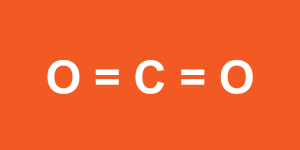
The polarity of a molecule is determined by the polarity of the covalent bonds within the molecule as well as the way the bonds are arranged. For example, carbon dioxide (CO2) is a nonpolar molecule, despite containing two atoms with very different electronegativities. In CO2, carbon forms two double bonds to each of the two oxygen atoms. Because the electron pairs strongly repel one another, CO2 takes on a linear shape (Figure 1.3.1). The two oxygens face away from one another symmetrically, effectively cancelling the polarity of the C-O bonds. For this reason, CO2 is poorly soluble in aqueous solutions such as blood plasma. You will learn in a future lecture on blood that CO2 must be converted into a soluble compound called carbonic acid (H2CO3) by the action of an enzyme called carbonic anhydrase in red blood cells.
Weaker interactions
The chemical bonds described above connect atoms within molecules or compounds. However, molecules can also interact with one another through chemical bonds, and these interactions are collectively known as intermolecular bonds, such as hydrogen bonds and hydrophobic interactions.
Hydrogen bonds
Electrostatic attraction between the partial negative charge (δ-) on the oxygen of one water molecule and the partial positive charges (δ+) on the hydrogens of another water molecule will be weakly attracted to one another. These interactions are relatively weak because the charges on the atoms are only partial charges. These weak intermolecular interactions that hold polar molecules together are called hydrogen bonds.
Hydrophobic interactions
Noncovalent interactions can occur between nonpolar molecules as well. Polar molecules interact favourably with other polar molecules. Therefore, polar molecules will arrange themselves to exclude nonpolar molecules in solution. These interactions will result in the association of nonpolar molecules with one another in aqueous solution, forming hydrophobic interactions. Though noncovalent, hydrophobic interactions are strong forces between molecules. In cells, hydrophobic interactions drive protein folding.
Nonpolar molecules are also held together by weak forces. Because electrons move quickly around the nucleus, there may be momentary differences in charge distribution within the electron clouds of atoms. This results in weak positive and weak negative areas of the electron cloud. The weakly positive portions of the electron cloud of one atom may be attracted to the weakly positive portions of another atom’s electron cloud. These momentary electrostatic interactions between nonpolar molecules are called van der Waal’s forces. Though individually weak, large nonpolar molecules may interact via hundreds of van der Waal’s forces and, collectively, these forces form strong interactions between molecules. In cells, van der Waal’s forces hold the nonpolar portions of lipids in cellular membranes together.
Chemical bonds hold atoms within a molecule together as well as molecules to other molecules. As a result, molecules within cells are arranged in a specific manner to reflect the function of those molecules within those organelles and cells.
Section Summary
- Atoms are held together in molecules by chemical bonds.
- The electronegativity of atoms affects the chemical properties of the bonds those atoms form with other atoms to form molecules.
- Bonds may be covalent or noncovalent.
- Covalent bonds involve the sharing of a pair of electrons.
- Polar covalent bonds form between atoms with different electronegativities.
- Nonpolar covalent bonds form between atoms with similar electronegativities.
- Noncovalent bonds include:
- Ionic bonds where one atom transfers an electron to another more electronegative atom.
- Hydrogen bonds where one polar molecule is attracted to the opposite partial charges on another polar molecule.
- Strong hydrophobic interactions between nonpolar molecules.
- Weak van der Waal’s forces between nonpolar molecules.
- Covalent bonds involve the sharing of a pair of electrons.
Water
Water is essential for life. Life likely originated in water and water on Earth’s surface continues to make Earth habitable. Water serves as a solvent for the chemical reactions inside and outside of cells that support life and water constitutes 60–90% of cell mass. In the human body, most metabolic reactions happen in water and water serves as a lubricant in body fluids. In this section, we explore how the chemical structure of water leads to its remarkable chemical properties that sustain life.
The structure of water
Oxygen is more electronegative than hydrogen; therefore, polar covalent bonds hold the atoms within water molecules together. The oxygen atom pulls with greater force on the shared electrons with hydrogen, resulting in a partial negative charge (δ-) on the oxygen atom. Each of the hydrogen atoms possess partial positive charges (δ+). In a solution of pure water, the partial negative charge on the oxygen is attracted to the partial positive charges on other water molecules, forming hydrogen bonds between water molecules. The hydrogen bonds between water molecules are responsible for all the properties of water.
The properties of water include:
- Cohesion
- High heat capacity
- Expansion upon freezing
- Versatility as a solvent
Water molecules exhibit cohesion
The hydrogen bonds between water molecules are weak relative to covalent bonds. However, water molecules tend to be attracted to other water molecules to form hydrogen bonds. The tendency for molecules to be attracted to one another if they are the same kind of molecules is called cohesion.
Cohesion allows water molecules to “stick together” via hydrogen bonding. Imagine a water droplet on the surface of your desk or table. Water droplets form because water molecules tend to be attracted to one another through hydrogen bonds rather than to your desk or table or to the air surrounding the water. This creates surface tension where water meets another type of matter. Water droplets form because of the cohesive properties of water!
In the human body, cohesion of water molecules ensures that when water moves in and out of thin-walled blood vessels called capillaries, one water molecule will be followed by many water molecules. This bulk movement of water ensures fluid balance within the body.
Water has a high heat capacity
Recall that the three states of matter include solid, liquid, and gas. When you think of water, you probably think of water in its liquid state. When water is boiled, the liquid is heated into a gas called water vapour. The water molecules move very fast in water vapour as compared to liquid water. When water is frozen, the liquid is cooled into ice. The water molecules in ice move much more slowly than in liquid water.
Recall that energy is the capacity to do work. Heat and temperature are two different measures of energy. Heat is the total kinetic energy within a system. Temperature is the average kinetic energy of a system. You may find it easier to understand the difference between these two measures of energy using the following example.
Water requires a large investment of heat energy before a small change in water temperature can occur. In other words, water has a high heat capacity. Imagine that you are boiling water to make tea. The amount of heat required to boil water is the total amount of energy you need to put in to raise the temperature of water to ~100°C. Water seemingly takes a long time to boil because energy must be invested to break the hydrogen bonds that hold water molecules together before those water molecules can move freely and form water vapour. Once the hydrogen bonds have been broken, individual water molecules move faster (gain kinetic energy), resulting in a change in temperature.
Similarly, boiled water takes a seemingly long time to cool. Once you have brewed your tea, you must wait for the tea to reach a drinkable temperature. As water cools, the molecules slow their movement and energy is released as the hydrogen bonds between water molecules re-form. That energy keeps the temperature of water relatively constant, even though you have stopped heating the water.
The high heat capacity of water makes Earth habitable. Heat from the atmosphere is absorbed by water in oceans, ensuring that the temperature on Earth’s surface does not exceed a livable temperature. Water can release heat to cooler atmospheric air, again, keeping the temperature on Earth’s surface relatively constant. Life on Earth is possible because of the ability of water molecules to form hydrogen bonds!
Water expands upon freezing
For most substances, the solid state of matter is denser than the liquid state of the same substance. As atoms and molecules slow their movement at low temperature, they get closer together. Generally, this increases the density of a substance.
Water is one exception to this rule. In liquid water, some molecules are bound together by hydrogen bonds but some molecules move freely. As liquid water freezes and hydrogen bonds form between water molecules, those hydrogen bonds form a geometrical pattern called a lattice. The fixed geometry of the lattice prevents water molecules from packing close together and, therefore, from becoming denser as it freezes. Instead, water expands upon freezing.
This is why ice floats in cold drinks and why liquid beverages cooled in the freezer may explode. Similarly, in the polar regions of the globe (high latitudes), ice forms over parts of the polar seas during cold seasons. The diverse marine life that lives in those oceans can survive cold seasons because ice floats on top and insulates the deeper water against colder atmospheric air.
Water is a versatile solvent
Water is the solvent for most of life’s chemical reactions. A solution is a uniform mixture of liquid and substances dissolved in that liquid. A solution consists of a solvent or liquid and a solute or substance dissolved in the solvent.
Solution = solute(s) + solvent
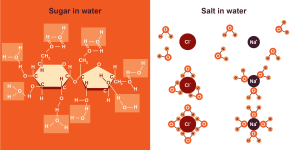
Water is a polar molecule and a polar solvent. This means that water will dissolve other polar molecules as well as charged molecules. One example of a polar molecule is table sugar or sucrose (C12H22O11). Sucrose stirred into water dissolves easily because both sucrose and water are polar molecules and the partial charges on the atoms of those molecules interact with one another favourably. The covalent bonds within sucrose are not easily weakened by water and remain intact when sucrose dissolves.
Charged molecules, such as table salt or sodium chloride (NaCI) also interact favourably with water. The partial negative charges on the oxygen atoms of water molecules attract the positively charged sodium ions; the partial positive charges on the hydrogen atoms of water molecules attract the negatively charged chloride ions. Because the partial charges of water can disrupt the electrostatic interactions between ions, ionic bonds are easily weakened by water. Therefore, water molecules tend to disrupt ionic bonds between ions and surround ions in solution, forming a hydration sphere.
Substances that interact favourably with water are called hydrophilic. Polar and charged solutes are hydrophilic and tend to dissolve easily in water.
In contrast, nonpolar molecules bear no partial charges or full charges to interact favourably with partial charges on water molecules. Therefore, nonpolar molecules are called hydrophobic. Nonpolar solutes do not dissolve in water easily.
Chemistry in the clinic:
Colloids are suspensions of substances in a solvent-like liquid. Colloids differ from solutions by the size of the particle in the liquid. If the substance contains particles that measure between 1 nm and 1 μm (~ size of a bacterial cell), then the mixture is a colloid and not a solution. If the particles are smaller than 1 nm, then the mixture is a solution. Blood plasma, the liquid component of blood, is a colloid that contains relatively large proteins that are required for clotting. You will learn about the consequences of the colloidal nature of blood in a future lecture on the vascular system and hemodynamics.
1 nm = 1 x 10-9 m
1 μm = 1 x 10-6 m
Section Summary
- Water is essential for cell function and life on Earth.
- The atoms in a water molecule are held together by polar covalent bonds.
- Molecules of water are held together by hydrogen bonds.
- The hydrogen bonds of water are responsible for its remarkable properties, including:
- Cohesion
- Ability to moderate temperature
- Expansion upon freezing
- Versatility as a solvent
Charged atoms
Tendency for molecules of the same type to hold tightly to one another rather than spread out
the energy of movement
Derived from Greek: “hydros” = water; “philos” = love; water-loving
Derived from Greek: “hydros” = water; “phobos” = fear; water-fearing

