6.5 Fermentation
Learning Objectives
By the end of this section, you will be able to:
- Discuss the fundamental difference between anaerobic cellular respiration and fermentation
- Describe the type of fermentation that readily occurs in animal cells and the conditions that initiate that fermentation
In aerobic respiration, the final electron acceptor is an oxygen molecule, O2. If aerobic respiration occurs, then ATP will be produced using the energy of the high-energy electrons carried by NADH or FADH2 to the electron transport chain. If aerobic respiration does not occur, NADH must be reoxidized to NAD+ for reuse as an electron carrier for glycolysis to continue.
Some living systems use an organic molecule (e.g. lactic acid) as the final electron acceptor. Processes that use an organic molecule to regenerate NAD+ from NADH are collectively referred to as fermentation. In contrast, some living systems use an inorganic molecule as a final electron acceptor; both methods are a type of anaerobic cellular respiration. Anaerobic respiration enables organisms to convert energy for their use in the absence of oxygen.
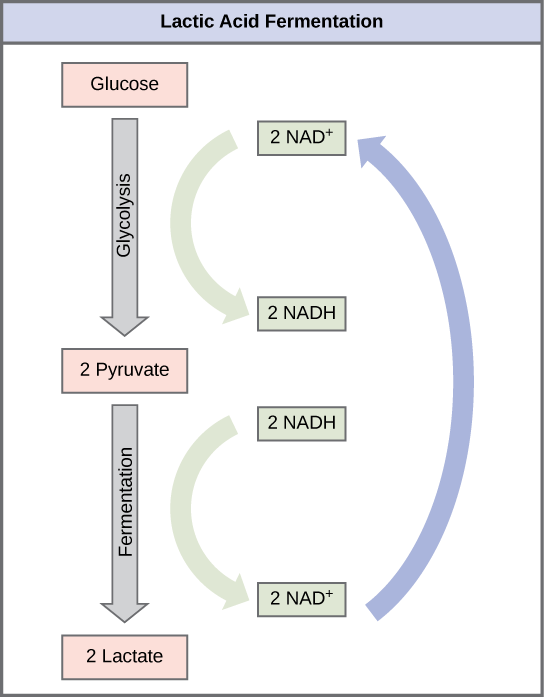
Lactic Acid Fermentation
The fermentation method used by animals and some bacteria is lactic acid fermentation (Figure 6.5.1). This occurs routinely in mammalian erythrocytes and in skeletal muscle that has insufficient oxygen supply to allow aerobic respiration to continue (that is, in muscles used to the point of fatigue). In muscles, lactic acid produced by fermentation must be removed by the blood circulation and brought to the liver for further metabolism. The enzyme that catalyzes this reaction is lactate dehydrogenase. A build-up of lactic acid causes muscle stiffness and fatigue. Once this lactic acid has passed from the muscle to the liver, it can be converted back to pyruvate and further catabolized for energy. This process by which lactic acid produced in anoxic muscle cells is converted back to glucose in the liver is called the Cori cycle.
Visual Connection
Alcohol Fermentation
Another familiar fermentation process is alcohol fermentation (Figure 6.5.2), which occurs in yeast resulting in the production of ethanol. The alcohol fermentation reaction is the following:
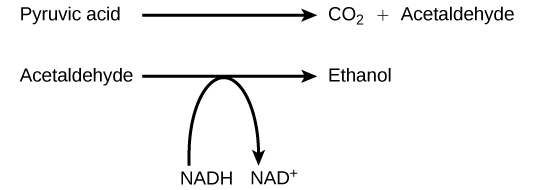
In the first reaction, a carboxyl group is removed from pyruvic acid (pyruvate), releasing CO2 as a gas. The loss of CO2 reduces the molecule by one carbon atom, making acetaldehyde. The second reaction removes an electron from NADH, forming NAD+ and producing ethanol from the acetaldehyde, which accepts the electron. The fermentation of pyruvic acid by yeast produces the ethanol found in alcoholic beverages (Figure 6.5.3). If the CO2 produced by the reaction is not vented from the fermentation chamber, for example in beer and sparkling wines, it remains dissolved in the medium until the pressure is released. Ethanol above 12% is toxic to yeast, so natural levels of alcohol in wine occur at a maximum of 12%.

Look at measuring respiration in yeast to see anaerobic cellular respiration in action.
Section Summary
- If NADH cannot be metabolized through aerobic respiration, another electron acceptor is used.
- Most organisms will use some form of fermentation to accomplish the regeneration of NAD+, ensuring the continuation of glycolysis. The regeneration of NAD+ in fermentation is not accompanied by ATP production.
Glossary
anaerobic cellular respiration: the use of an electron acceptor other than oxygen to complete metabolism using electron transport-based chemiosmosis
fermentation: the steps that follow the partial oxidation of glucose via glycolysis to regenerate NAD+; occurs in the absence of oxygen and uses an organic compound as the final electron acceptor

