3.5 The Carbonyl Group
Learning Objectives
By the end of this section, you will be able to:
- Identify and describe carbonyl compounds
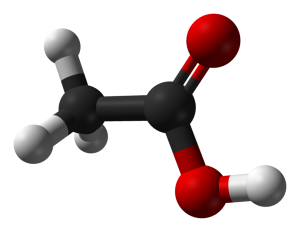
Double bonds between carbon and oxygen are common in nature. Organic families containing this arrangement of atoms are referred to as ‘carbonyl compounds’ and the group itself is referred to as a ‘carbonyl group.’ Multiple organic families include this arrangement of atoms, and each has its own distinct characteristics. Included in the larger group of carbonyl compounds are the narrower families: aldehydes, ketones, carboxylic acids, esters and amides. Others also exist.
The carbonyl group utilizes two of carbon’s four valence shell electrons for bonding, resulting in a trigonal planar arrangement around the carbon. As a result, the bond angles between the carbonyl carbon and each of the other atoms it is attached to is 120 degrees. The carbon to oxygen bond is polar, with a partial positive charge at the oxygen. Additionally, that oxygen includes two non-bonding, lone pairs of electrons. This concentration of electron charge at the oxygen results in significant dipole-dipole interactions in samples of most carbonyl compounds.
Some carbonyl compounds may be able to engage in hydrogen bonding interactions as well, but not all do. So, the families within this larger group have noticeable differences that relate to this fact.
Practice Questions
Section Summary
- Carbonyl compounds, characterized by a double bond between carbon and oxygen, include various organic families such as aldehydes, ketones, carboxylic acids, esters, and amides. Each family has distinct properties, but they all share the carbonyl group with a polar C=O bond and dipole-dipole interactions.
- The carbonyl group has a trigonal planar structure around the carbon atom, with bond angles of 120 degrees. The oxygen is highly electronegative, contributing to significant dipole-dipole interactions in most carbonyl compounds. Some carbonyl compounds can also engage in hydrogen bonding, though not all do.

