3.5.2 The Carbonyl Group: Carboxylic Acids
Learning Objectives
By the end of this section, you will be able to:
- Identify the carboxylic acid functional group
- Apply IUPAC nomenclature for carboxylic acids
- Explain the chemical properties and acidity of carboxylic acids
The carboxylic acid functional group includes a carbonyl carbon connected directly to an alcohol group, as shown below. Carboxylic acids are common in nature. Their properties are quite different from the other carbonyl compounds mentioned so far. Notably, as the name suggests, carboxylic acids are acidic.
Many carboxylic acids are known by their common names, including lactic acid, citric acid, glutamic acid and others. Systematic names for these substances also exist. They modify the name of the corresponding hydrocarbons to include the suffix ‘-oic acid.’ For instance:
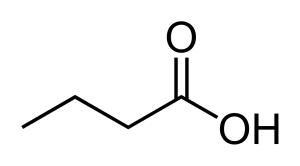
This structure is butanoic acid.
As with some other organic families, common names are very frequently used with carboxylic acids, and some are similar to, but not quite aligned with, IUPAC rules. This is difficult for students, but sometimes, enough information from the IUPAC system is included in a common name to allow you to decipher the structure.
To illustrate, the carboxylic acid above is often called by its common name ‘butyric acid.’ Butyric comes from the ancient Greek word for butter, the source from which this compound was first isolated. Butyric acid is a breakdown product of the fats in milk that is actually associated with spoilage in butter and cheeses, and while it contributes to flavour in some foods (e.g. Parmesan cheese), it also occurs in stale perspiration and vomit!

Similar to the situation for aldehydes, the carboxylic acid group always exists at the end of the parent chain because the functional group requires 3 of 4 available valence electrons on the carbonyl carbon. The carbonyl carbon in these molecules is designated as carbon 1, so location signifiers are not required to pinpoint the location of a carboxylic acid group unless a name describes a complex substance with more than one of these groups on it.
Both oxygen atoms on the carbonyl carbon in a carboxylic acid are electronegative, drawing electron density away from surrounding atoms toward themselves. The hydrogen atom is acidic due to the stability of the anion that exists when that hydrogen ion dissociates from the larger structure.

Carboxylic acids in water solution exist in an equilibrium between the acid and conjugate base states, shown here as the reactant and the anion product of this reaction. The double-headed arrow shown in the figure indicates the nature of this reaction is an equilibrium, with both forward and reverse reactions occurring to some degree. Carboxylic acids tend to be mostly in the reactant form at physiological pH, with perhaps 5 % of the substance in a deprotonated anion form at any given moment.
This amount of acid added to a solution from this type of dissociation of a carboxylic acid, however, is significant in many contexts. For instance, the acidity of ethanoic acid, also known as acetic acid, gives vinegar solutions their sour taste and contributes to their ability to retard the growth of undesirable organisms in pickles. Lactic acid in yogurt is another example of a carboxylic acid that has a big influence on its medium, as milk is converted by lactic acid-forming bacteria to kefir or yogurt.
Practice Questions

All of the substances in the interactive questions below are present in many ripe cheeses, including the famously funky cheese named Camembert.
Section Summary
- The carboxylic acid group consists of a carbonyl carbon bonded to a hydroxyl group. This functional group imparts distinct properties, particularly its acidity, setting it apart from other carbonyl compounds. Carboxylic acids are common in nature and can be named systematically by adding the suffix ‘-oic acid’ to the corresponding hydrocarbon name.
- Carboxylic acids are often referred to by their common names, such as lactic acid or citric acid, though systematic IUPAC names exist. For example, butanoic acid is commonly known as butyric acid, derived from butter. Despite the frequent use of common names, systematic names can help decode the structure of these compounds.
- The carboxylic acid group is always positioned at the end of the carbon chain, with the carbonyl carbon designated as carbon 1. As a result, location numbers are typically unnecessary unless the compound contains multiple carboxyl groups. The two oxygen atoms in the carboxyl group are highly electronegative, contributing to the compound’s acidic nature.
- Carboxylic acids in aqueous solutions exist in equilibrium between their acidic form and conjugate base. The dissociation of the hydrogen ion from the carboxyl group makes the substance acidic. Examples include acetic acid in vinegar and lactic acid in yogurt, which influence the properties and characteristics of their respective environments.

