6.2 Glycolysis
Learning Objectives
By the end of this section, you will be able to:
- Explain how ATP is used by the cell as an energy source
- Describe the overall result in terms of molecules produced of the breakdown of glucose by glycolysis
Energy production within a cell involves many coordinated chemical pathways. Most of these pathways are combinations of oxidation and reduction reactions. An oxidation reaction strips an electron from an atom in a compound, and the addition of this electron to another compound is a reduction reaction. Because oxidation and reduction usually occur together, these pairs of reactions are called oxidation-reduction reactions, or redox reactions.
Electrons and Energy
The removal of an electron from a molecule, oxidizing it, results in a decrease in potential energy in the oxidized compound. This electron is shifted to a second compound, reducing it in the process. The shift of an electron from one compound to another removes some potential energy from the first compound (the oxidized compound) and increases the potential energy of the second compound (the reduced compound). The transfer of electrons between molecules is important because most of the energy stored in atoms and used to fuel cell functions is in the form of high-energy electrons. The transfer of energy in the form of electrons allows the cell to transfer and use energy in an incremental fashion—in small packages rather than in a single, destructive burst.
Electron Carriers
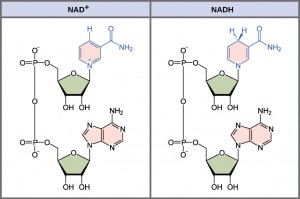
In living systems, a small class of compounds function as electron shuttles: they bind and carry high-energy electrons between compounds in different metabolic pathways. These compounds can be easily reduced (that is, they accept electrons) or oxidized (they lose electrons). Nicotinamide adenine dinucleotide (NAD) (Figure 6.2.1) is derived from vitamin B3 (niacin). A second variation of NAD, NADP, contains an extra phosphate group. NAD+ is the oxidized form of the molecule; NADH is the reduced form of the molecule after it has accepted two electrons and a proton (which together are the equivalent of a hydrogen atom with an extra electron which is called a hydride ion).
NAD+ can accept electrons from an organic molecule according to equation [3]:
RH (Reducing Agent) + NAD+ (Oxidizing Agent) —-> NADH (Reduced) + R (Oxidized). [3]
When electrons are added to a compound, they are reduced. A compound that reduces another is called a reducing agent. In the above equation, RH is a reducing agent, and NAD+ is reduced to NADH. When electrons are removed from compound, it is oxidized. A compound that oxidizes another is called an oxidizing agent. In the above equation, NAD+ is an oxidizing agent, and RH is oxidized to R.
Similarly, flavin adenine dinucleotide (FAD+) is derived from vitamin B2 (also called riboflavin). Its reduced form is FADH2. Both NAD+ and FAD+ are extensively used in energy extraction from sugars, while NADP plays an important role in anabolic reactions and photosynthesis.
ATP in Living Systems
Living cells do not transfer energy in the form of heat. The amount of energy released from some reactions are so large that if it was released as heat the cell would vaporise as a result. Cells use high energy containing compounds to store the energy safely and release it for use only as needed. The main high energy containing compound found in the cell is adenosine triphosphate or ATP.
When ATP is broken down, usually by the removal of its terminal phosphate group, energy is released. The cell uses the energy to do work, usually by the released phosphate binding to another molecule, activating it. For example, in the mechanical work of muscle contraction, ATP supplies the energy to move the contractile muscle proteins. Recall the active transport work of the sodium-potassium pump in cell membranes. ATP alters the structure of the integral protein that functions as the pump, changing its affinity for sodium and potassium. In this way, the cell performs work, pumping ions against their electrochemical gradients.
ATP Structure and Function
At the heart of ATP is a molecule of adenosine monophosphate (AMP), which is composed of an adenine base bonded to a ribose molecule and a single phosphate group (Figure 6.2.2). Ribose is a five-carbon sugar found in RNA, and AMP is one of the nucleotides in RNA. The addition of a second phosphate group to this core molecule results in the formation of adenosine diphosphate (ADP); the addition of a third phosphate group forms adenosine triphosphate (ATP).
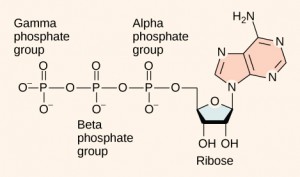
The addition of a phosphate group to a molecule requires energy. The release of one or two phosphate groups from ATP, a process called dephosphorylation, releases energy.
Glycolysis
You have read that nearly all of the energy used by living things comes to them in the bonds of glucose. Glycolysis is the first step in the breakdown of glucose to extract energy for cell metabolism. Many living organisms carry out glycolysis as part of their metabolism. Glycolysis occurs in the cytosol of most prokaryotic and all eukaryotic cells.
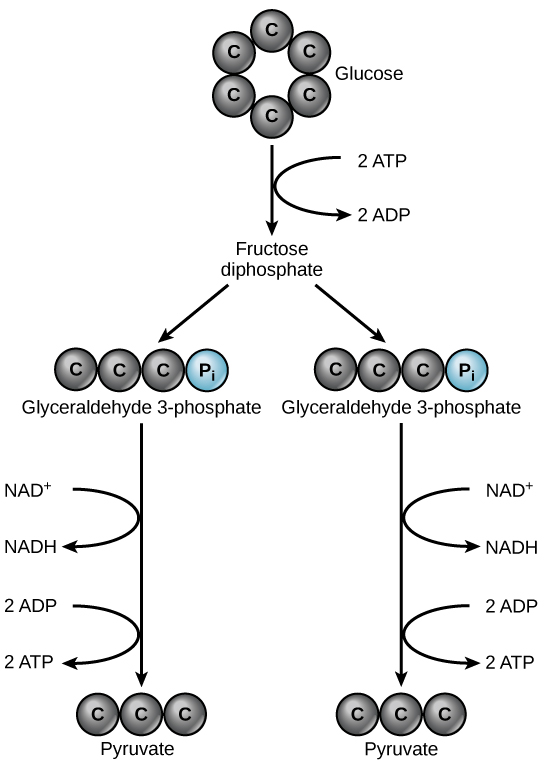
Glycolysis begins with the six-carbon, ring-shaped structure of a single glucose molecule and ends with two molecules of a three-carbon sugar called pyruvate. Glycolysis consists of two distinct phases. In the first part (preparatory phase) pathway, energy (2 ATP molecules) is used to make adjustments so that the six-carbon sugar molecule can be split evenly into two three-carbon pyruvate molecules. In the second part of glycolysis (payoff phase), ATP (4 ATP) and nicotinamide-adenine dinucleotide (NADH) (2 NADH) are produced (Figure 6.2.3).
If the cell cannot further catabolize pyruvate, it will harvest only two ATP molecules from one molecule of glucose. For example, mature mammalian red blood cells are only capable of glycolysis, which is their sole source of ATP. If glycolysis is interrupted, these cells would eventually die.
Section Summary
- ATP functions as the energy currency for cells. It allows cells to store energy briefly and transport it within itself to support endergonic chemical reactions. The structure of ATP is that of an RNA nucleotide with three phosphate groups attached. As ATP is used for energy, a phosphate group is detached, and ADP is produced. Energy derived from glucose catabolism is used to recharge ADP into ATP.
- Glycolysis is the first pathway used in the breakdown of glucose to extract energy. Because it is used by nearly all organisms on earth, it must have evolved early in the history of life. Glycolysis consists of two parts: The first part prepares the six-carbon ring of glucose for separation into two three-carbon sugars. Energy from ATP is invested into the molecule during this step to energize the separation. The second half of glycolysis extracts ATP and high-energy electrons from hydrogen atoms and attaches them to NAD+. Two ATP molecules are invested in the first half and four ATP molecules are formed during the second half. This produces a net gain of two ATP molecules per molecule of glucose for the cell.
Glossary
ATP: (also, adenosine triphosphate) the cell’s energy currency
glycolysis: the process of breaking glucose into two three-carbon molecules with the production of ATP and NADH

