Colour chemistry 4: fireworks

How do they make different coloured fireworks?
Fireworks were recorded as originating from China in the 8th Century CE. It is said that explosive black powder was accidentally invented by alchemists who mixed potassium nitrate (also called saltpetre), sulfur and honey together while trying to create the elixir of life. The mixture exploded when heated – this is called an exothermic chemical reaction. Recipes for this explosive mixture eventually made their way across Asia and the Arabic world to Europe, which is where fireworks as we know them today were first created.
The explosive part of fireworks is gunpowder (black powder) which is now made from saltpetre, sulfur and charcoal. The reason fireworks can appear to have a range of colours is because different chemical compounds are used, which are mostly metals that burn very brightly, and these are coated in gunpowder. Exploding gunpowder burns the chemical compounds, which causes electrons in the compounds to get excited and release excess energy as photons (light). These photons have different wavelengths in the visible spectrum depending on the energy of each chemical’s electrons, and this is how we see fireworks as different colours (Figure 2.41).
Some colours, like blue fireworks, are harder to make because the chemical compounds are not stable. Purple is also difficult because it’s made from a mixture of red and blue compounds.
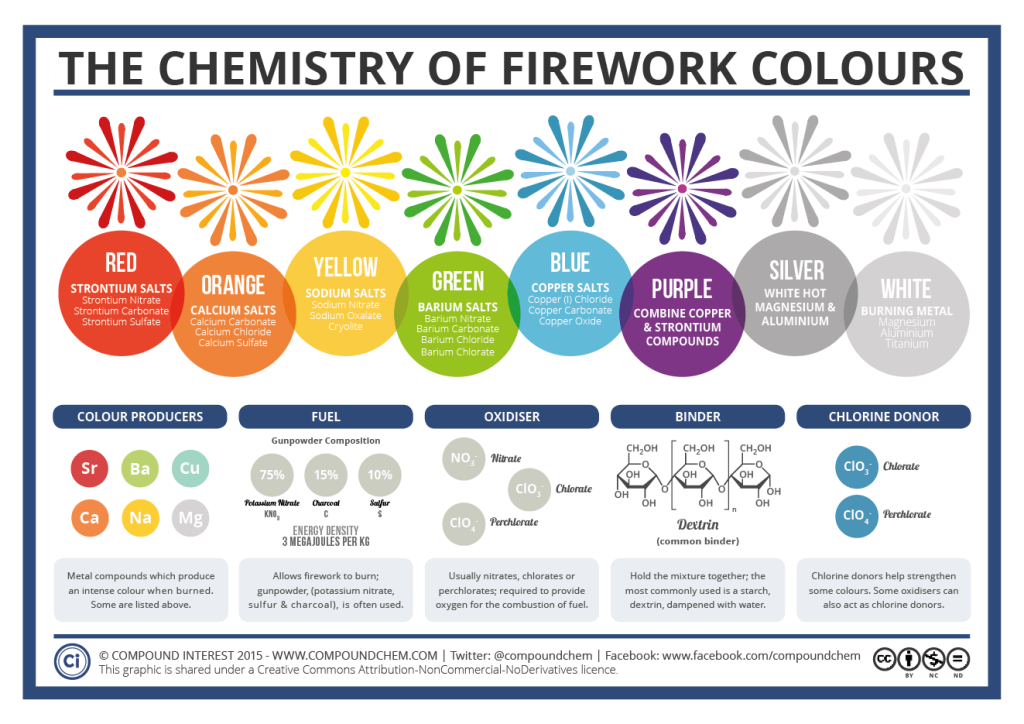
Fireworks colours and their chemical compounds:
- Red – Strontium salts
Strontium Nitrate, Strontium Carbonate and Strontium Sulfate - Orange – Calcium salts
Calcium Carbonate, Calcium Chloride and Calcium Sulfate - Yellow – Sodium salts
Sodium Nitrate, Sodium Oxalate and Cryolite - Green – Barium salts
Barium Nitrate, Barium Carbonate, Barium Chloride and Barium Chlorate - Blue – Copper salts
Copper (I) Chloride, Copper Carbonate and Copper Oxide - Purple – Combination of Red and Blue
Strontium and Copper compounds - Silver – white hot metals
Magnesium and Aluminium - White – burning metal
Magnesium, Aluminium and Titanium