Colour chemistry 3: universal indicator and diagnostic tests

Universal indicator
We can identify the acidity levels (pH value) of many substances with colour testing.
What is pH?
pH, originally meaning “potential of Hydrogen”, is a numbering system used for measuring the acidity of substances dissolved in a water solution. A low pH value has a higher acid content. A high pH value indicates a higher alkaline or basic substance. Water has a neutral pH value of 7, which is why it’s a good solution to test the pH of other substances dissolved in it. For example, lemon juice can have a pH of around 2 – which is very acidic. Lemons have a high quantity of citric acid in them, which is why they have a low pH value. The detergent you use to wash your dishes is alkaline or basic, so it might have a pH value of around 8.
Learn more from this book Acids and Alkalis by Denise Walker.
To test the pH level of any solution, a universal indicator is used. This can be in the form of a paper tape that you dip into your solution, or it could be a liquid that is added to a solution. The universal indicator contains a set of chemicals that change colour depending on the amount of acid or base substances in the solution (Figure 2.37). They work by producing extra protons depending on how acidic or alkaline the solution is, which in turn emit photons with different wavelengths.
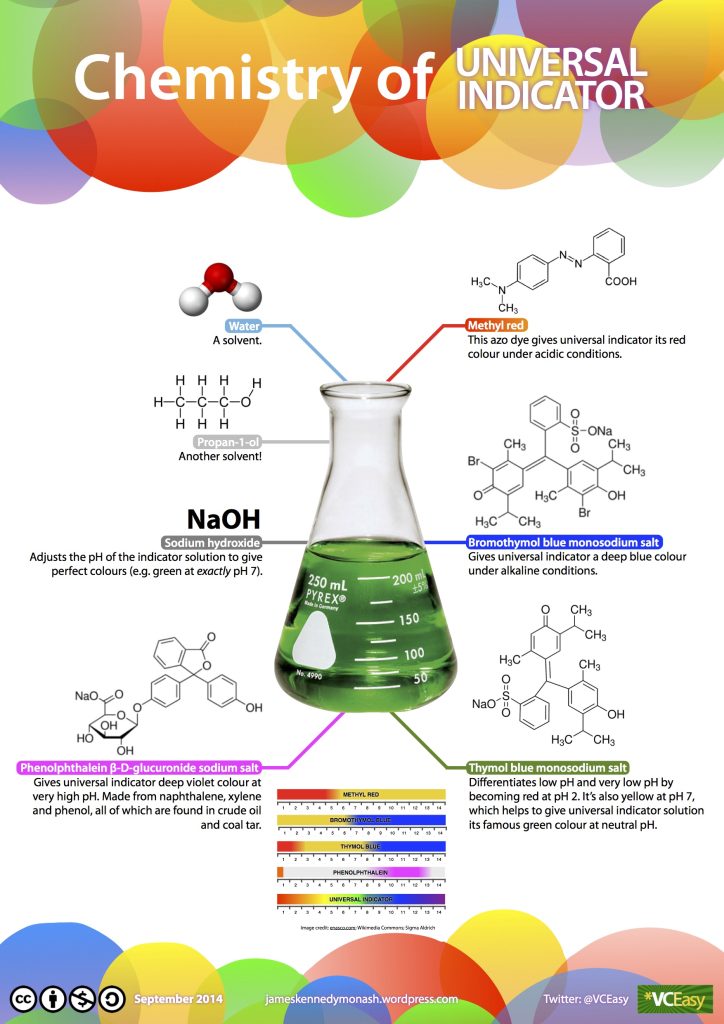

You can also use red cabbage as an indicator by making a solution with chopped-up leaves and water. Red cabbage contains a water-soluble pigment called anthocyanin, which turns red if mixed with an acid substance and blue/green if mixed with an alkaline/base substance.
There are other plants that have anthocyanin and can also be used in the same way. Can you find three other examples?
Learn more about acids and bases.
You can buy universal indicator online and do your own experiments with substances around your home to see which are acids and which are bases, or you can make your own indicator with red cabbage. You can start by testing foods, drinks and cleaning products around your home. Is your tap water a pH of 7? If not, it might have other chemicals in it. See the activity below for instructions on making your own red cabbage indicator.
Activity: make a red cabbage indicator
You can make your own indicator at home with a red cabbage and a few simple materials to test the pH of different substances.
Watch this video for details and try your own experiments:
Diagnostic medical tests
Have you ever wondered how a pregnancy test or a COVID Rapid Antigen Test (RAT) works? How do the coloured lines appear on the test strip?
A pregnancy test uses chemicals that change colour when they come into contact with certain enzymes that attach to antibodies. When you are pregnant, your body produces a hormone called human chorionic gonadotropin (hCG). This hormone is excreted by the kidneys into urine – so putting urine on the test strip will start a chemical process that tests for the hCG hormone. The test strip contains an enzyme that will attach itself to the hCG hormone in the urine. As the urine moves along the test strip, there are two lines that can change colour – one will change colour and become visible if it comes into contact with the enzyme that is attached to the hCG – the test line. Another line will change colour and become visible if the enzymes not attached to hCG come into contact with it – the control line.
Two lines are needed to ensure the test has worked correctly because if there was only one line, it could show a false-negative result if the test didn’t work properly.
Watch this video that explains how a COVID rapid antigen test works:


