8.1 Introduction to Hydrocarbons
Learning Objectives
- Differentiate four classes of hydrocarbons.
- Explain saturated and unsaturated hydrocarbons and how their structures influence their reactivity.
Hydrocarbons are organic compounds that only contain carbon and hydrogen. They are used in a wide variety of applications and on an enormous scale. Hydrocarbons are sources from natural gas and crude oil, the fossil fuel substances taken from underground. These unrefined products are mixtures that are refined in industrial facilities to generate the raw materials used to produce everything from gasoline to plastics, paints, and cosmetics. Hydrocarbons are the simplest organic compounds, but they have interesting physiological effects. These effects depend on the size of the hydrocarbon molecules and the part of the body that is exposed to them.
Hydrocarbons are classified into four main classes: alkanes, alkenes, alkynes, and aromatic hydrocarbons (Figure 8.1.1):
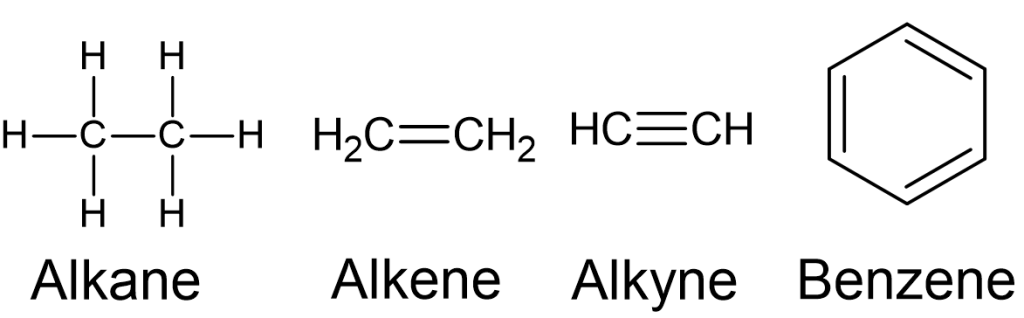
Alkanes
- Alkanes are comprised of [latex]\ce{C}-\ce{C}[/latex] single bonds and have no functional groups.
- They are known as saturated hydrocarbons due to the fact that these molecules have the maximum number of hydrogen atoms possible. In alkanes, each carbon atom forms four single covalent bonds, two with adjacent carbon atoms and two with hydrogen atoms. This results in a structure where each carbon is "saturated" with the maximum number of hydrogen atoms it can hold.
- Methane (Figure 8.1.1) is the simplest alkane, and its molecular formula is [latex]\ce{CH_{4}}[/latex].
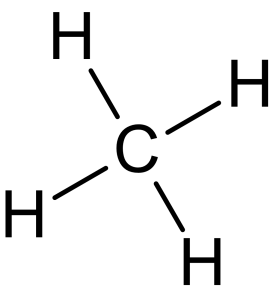
- The general formula for alkanes is [latex]\ce{C_{n}H_{2n+2}}[/latex]. This formula indicates that for every [latex]n[/latex] carbon atom, there are [latex]2n+2[/latex] hydrogen atoms. This ensures that each carbon atom is bonded to four other atoms (either other carbon atoms or hydrogen atoms), satisfying the tetravalency of carbon.
- Alkanes can form straight-chain, branched and cyclic structures.
- Straight-chain alkanes: they are distinguished by the arrangement of carbon atoms in a continuous, unbranched chain, as shown in Figure 8.1.3.
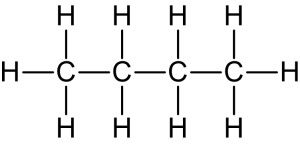
-
- Branched alkanes: alkanes can have branching, meaning that instead of forming a straight chain, some carbon atoms may have additional carbon atoms attached to them, as shown in Figure 8.1.4.
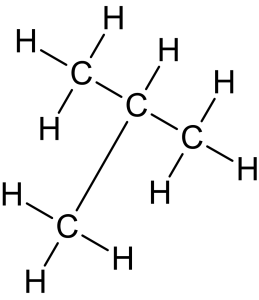
-
- Cyclic alkanes/cycloalkanes: in a cyclic alkane, the carbon atoms are arranged in a ring. The simplest example is cyclopropane [latex]\ce{C_{3}H_{6}}[/latex] is shown in Figure 8.1.5.
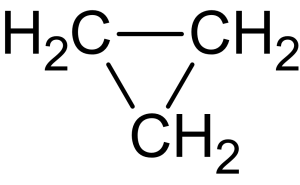
- Alkanes are known to be less reactive compared to other classes of organic compounds due to:
- strong single bonds between [latex]\ce{C}-\ce{C}[/latex] and [latex]\ce{C}-\ce{H}[/latex] and,
- the polarity of [latex]\ce{C}-\ce{C}[/latex] and [latex]\ce{C}-\ce{H}[/latex] bonds. In alkanes, the electrons in single bonds are approximately evenly distributed between the two bonded atoms, making them non-polar. Therefore, no significant positive or negative charge is present on any part of these molecules to attract other ions or molecules. Hence, alkanes are less susceptible to attack by reagents.
- Despite their general lack of reactivity, alkanes can undergo certain reactions under specific conditions. For example, they can undergo combustion (reaction with oxygen to produce carbon dioxide and water) and can be involved in radical reactions under the influence of high-energy conditions.
- Alkanes of low molar mass—those with from 1 to approximately 10 or so carbon atoms—are gases or light liquids that act as anesthetics. Methane, [latex]\ce{CH_{4}}[/latex], is an alkane that is the combustible natural gas you may burn in your furnace to heat your home. Octane, [latex]\ce{C_{8}H_{18}}[/latex], is an alkane that is a component of gasoline. On the skin, liquid alkanes with approximately 5–16 carbon atoms per molecule wash away natural skin oils and cause drying and chapping, while heavier liquid alkanes (those with approximately 17 or more carbon atoms per molecule) act as emollients (skin softeners).
Alkene
- Alkenes are a class of hydrocarbons characterised by the presence of at least one carbon-carbon double bond in their molecular structure.
- These compounds are part of the larger group of unsaturated hydrocarbons, as they contain fewer hydrogen atoms than the corresponding alkanes (saturated hydrocarbons) with the same number of carbon atoms.
- The general molecular formula for alkenes is [latex]\ce{C_{n}_H_{2n}}[/latex], where [latex]n[/latex] is the number of carbon atoms. The simplest alkene is ethylene with the molecular formula [latex]\ce{C_{2}H_{4}}[/latex] (Figure 8.1.6):
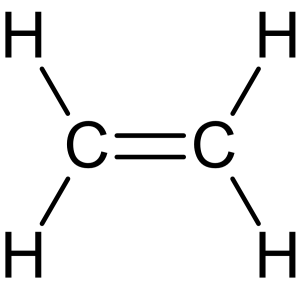
- The presence of a double bond introduces a degree of unsaturation into the molecule, allowing alkenes to undergo additional reactions not possible with saturated hydrocarbons.
- The carbon-carbon double bond consists of one sigma (σ) bond, which is formed by the head-on overlap of atomic orbitals, and one pi (π) bond, which is formed by the side-to-side overlap of p orbitals. The presence of the pi bond introduces a degree of rigidity and restricts rotation around the double bond.
- Alkenes are more reactive than alkanes due to the presence of the π bond. The π electrons are more accessible and can participate in various chemical reactions. Alkenes can undergo polymerisation to form polymers. For example, ethene can polymerise to form polyethylene, a widely used plastic.
- Ethene [latex]\ce{C_{2}H_{4}}[/latex] is a gaseous alkene that serves as a cellular signal in fruits to stimulate ripening. Fruits that are sensitive to this signalling molecule can be placed in a paper bag along with an apple - the apple emits ethene gas, setting off the ripening process in the fruit. Commercial fruit packers can make use of this phenomenon by harvesting unripe fruits and then inducing ripening right before shipping them to consumers.
- The bonding in alkenes is trigonal planar, and the molecules are unable to rotate along the axis of the bond. The double bonds thus lead to 120-degree bond angles and a planar triangular geometry around the double bond.
Alkynes
- Alkynes are a class of hydrocarbons characterised by the presence of at least one [latex]\ce{C}-\ce{C}[/latex] triple bond in their molecular structure. Like alkenes, alkynes are unsaturated hydrocarbons, meaning they have fewer hydrogen atoms than the corresponding alkanes with the same number of carbon atoms. The [latex]\ce{C}-\ce{C}[/latex] triple bond consists of one sigma (σ) bond and two pi (π) bonds. The triple bond imparts a degree of rigidity to the molecular structure.
- The general molecular formula for alkynes is [latex]\ce{C_{n}H_{2n-2}}[/latex], where [latex]n[/latex] is the number of carbon atoms. The simplest member of the alkyne family is ethyne with the molecular formula [latex]\ce{C_{2}H_{2}}[/latex] (Figure 8.1.7). The common name of ethyne is acetylene. Acetylene is widely used in oxy-acetylene welding and cutting processes. It is a precursor in the production of polymers. For example, it can polymerise to form polyacetylene, a conductive polymer with potential applications in electronic devices.

- Alkynes are more reactive than alkanes due to the presence of the triple bond and can undergo various chemical reactions like alkenes. The hydrogen atoms attached to the carbon atoms of the triple bond in alkynes are relatively acidic compared to those in alkanes and alkenes. This acidity is a result of the electronegative nature of the adjacent triple bond.
- In alkynes, the geometry around the triple bond is linear (bond angles are 180º), and only one other atom can bond to the alkyne carbon, so there is no rotation.
Aromatic Hydrocarbons
- Aromatic hydrocarbons are a class of organic compounds that contain a cyclic structure with alternating single and double bonds, known as an aromatic ring. In the aromatic ring, each carbon atom is bonded to two other carbon atoms and one hydrogen atom. The electrons in the pi (π) bonds are delocalised, creating a stable, resonant structure.
- Aromatic hydrocarbons are unsaturated, like alkenes and alkynes. Although aromatic hydrocarbons contain double bonds, their reactivity differs from that of typical alkenes. Aromatic compounds are known for their stability and resistance to certain reactions that would break the aromaticity of the ring.
- Benzene ([latex]\ce{C_{6}H_{6}}[/latex]) is the simplest and most well-known aromatic hydrocarbon (Figure 8.1.8). Other common aromatic hydrocarbons include toluene, xylene, naphthalene, and anthracene. Aromatic rings can also be found in many organic compounds, including certain classes of drugs, dyes, and natural products.
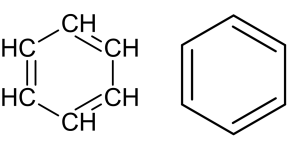
- Aromatic hydrocarbons have numerous industrial applications. They are key components in the production of plastics, synthetic fibres, detergents, pharmaceuticals, and many other chemicals.
- Aromatic groups are planar (flat) ring structures and are widespread in nature, so you will see them frequently if you encounter chemical structures in biology classes or in biomedical work.
Key Takeaways
- Hydrocarbons are organic compounds that only contain carbon and hydrogen.
- Hydrocarbons are classified into four main classes: alkanes, alkenes, alkynes, and aromatic hydrocarbons.
- Alkanes are comprised of [latex]\ce{C}-\ce{C}[/latex] single bonds, alkenes consist of one or more [latex]\ce{C}-\ce{C}[/latex] double bond and alkynes are made up of one or more [latex]\ce{C}-\ce{C}[/latex] triple bonds.
- Alkanes are classified as saturated hydrocarbons, while alkenes, alkynes and aromatic hydrocarbons are classified as unsaturated hydrocarbons.
- Alkanes are less reactive compared to other hydrocarbons due to the presence of strong [latex]\ce{C}-\ce{C}[/latex] and [latex]\ce{C}-\ce{H}[/latex] single bonds.
Exercises

