3.5 Intermolecular Forces
Learning Objectives
- Understand the types of intermolecular forces: dipole-dipole interactions, hydrogen bonding and London dispersion forces, and their relative strengths.
- Learn how to identify what attraction forces are likely to present within a given molecule depending on its structure.
The bonds we have just discussed (covalent, ionic and metallic) are all examples of intramolecular forces: the forces inside a molecule that keep it together. However, molecules commonly interact with each other. For instance, the formation of solids is due to the compounds being within close proximity to each other. What is holding these molecules together?
Dipole-Dipole Interactions and Hydrogen Bonding
Like the ends of a magnet, opposite charges attract, while similar charges repel. As a result, attraction forces between molecules are naturally present in those with charge. Recall that hydrogen chloride, a polar covalent molecule, experiences partial charges on its constituents. In this case, chlorine is more electronegative and is partially negative, given the closeness of the electron to the atom. On the other hand, hydrogen is partially positive because the electron is further away (see Figure 3.5.1).
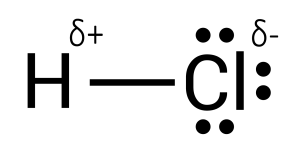
When a molecule of hydrogen chloride is surrounded by another molecule of hydrogen chloride, it will begin to align according to attraction forces. Opposite ends will neighbour each other, as seen in Figure 3.5.2.
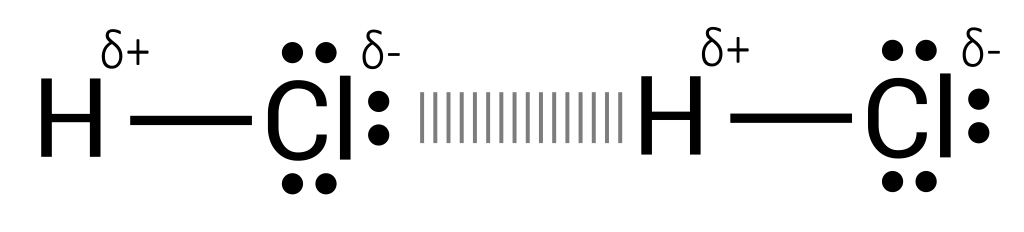
This is known as a dipole-dipole interaction – where molecules experience attraction and repulsion forces due to a permanent charge. Hydrogen bonds are a special kind of dipole-dipole interaction, as hydrogen exhibits unusually strong intermolecular forces when paired with a highly electronegative atom such as chlorine or oxygen (see Figure 3.5.3).
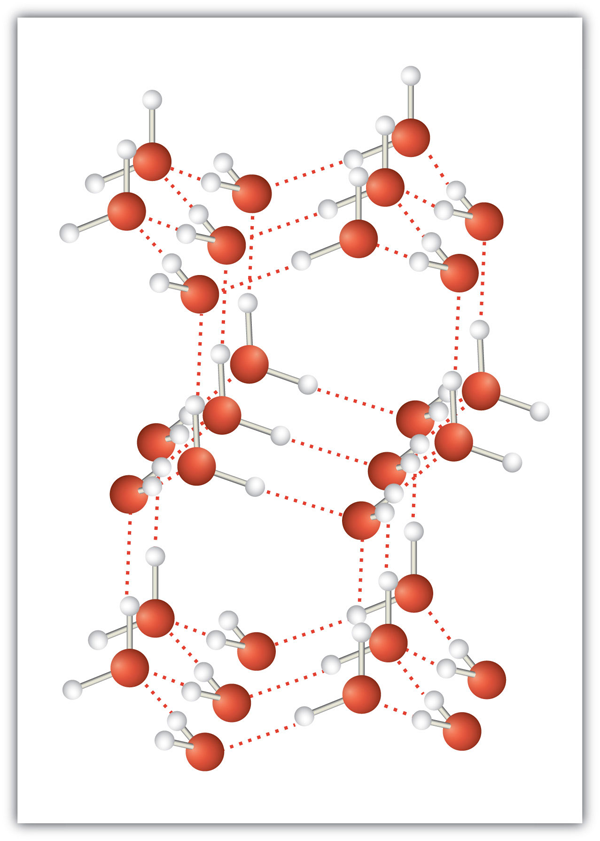
Intermolecular forces allow us to understand why molecules have a tendency to stay together (see Figure 3.5.3). It also explains the melting and boiling points of different compounds. Molecules that are closely attracted to one another require higher kinetic energy and, therefore, higher temperature to separate and undergo a phase change. The greater the strength of the attraction and the more interactions that are allowed to occur, the greater the energy requirement. This is why ionic compounds (particularly salts) generally have high melting points. For reference, sodium chloride has a melting point of 802°C and a boiling point of 1465°C!
London Dispersion Forces
Under this theory, we should expect that for non-polar covalent molecules that don’t hold permanent dipoles, melting and boiling points should be the same due to a lack of partial charge. However, as Table 3.5.1 demonstrates, boiling points do change with respect to the length of the molecule.
| Chemical Name | Molecular Formula | Boiling Point (°C) |
| Methane | [latex]\ce{CH_4}[/latex] | -161.5 |
| Ethane | [latex]\ce{C_2H_6}[/latex] | -88.6 |
| Propane | [latex]\ce{C_3H_8}[/latex] | -42.1 |
| Butane | [latex]\ce{C_4H_10}[/latex] | -0.5 |
| Pentane | [latex]\ce{C_5H_12}[/latex] | 36.1 |
| Hexane | [latex]\ce{C_6H_14}[/latex] | 68.7 |
| Heptane | [latex]\ce{C_7H_16}[/latex] | 98.4 |
| Octane | [latex]\ce{C_8H_18}[/latex] | 125.6 |
| Nonane | [latex]\ce{C_9H_20}[/latex] | 150.8 |
| Decane | [latex]\ce{C_10H_22}[/latex] | 174.1 |
All molecules, regardless of dipoles, are subject to dispersion forces (also known as London forces). These occur due to electrons continuously shifting within molecules. These electrons may occasionally generate partial charges, which induce electrons in other atoms to do the same, generating a slightly attractive force between molecules. As the surface area of a molecule increases, so does the occurrence of dispersion forces. As a result, longer-chain alkanes (listed in Table 3.5.1) possess higher boiling points.
Those are the three kinds of intermolecular forces: hydrogen bonding, dipole-dipole interactions and London dispersion forces. You can see them listed in Table 3.5.2 and their relative strengths. In addition to dictating boiling and melting points within molecules, they also play an important part in the solubility of molecules within water.
| Intermolecular Force | Type of Molecule | Strength of Attraction |
| Hydrogen Bonding | Molecules containing H-X[1] | Very Strong |
| Dipole-Dipole Interactions | Polar Molecules | Strong |
| (London) Dispersion Forces | All molecules. | Weak |
Key Takeaways
- Intramolecular forces are types of bonding (ionic, covalent and metallic), while intermolecular forces are the attraction and repulsion experienced between molecules.
- Dipole-dipole interactions are experienced between molecules with partial charges.
- Hydrogen bonding are a form of dipole-dipole interactions, but only feature on molecules with hydrogen next to a highly electronegative element.
- London dispersion forces are felt by all molecules.
Exercises
- X = Electronegative Atom (O, N, Cl, Br, F, etc.) ↵
Forces (bonds) within a molecule that hold it together.
Intermolecular force due to the dipole of a molecule interacting with another dipole.
Intermolecular force found between molecules with hydrogen attached to a highly electronegative element.
Intermolecular force experienced by all molecules due to a temporary dipole from electron movement.
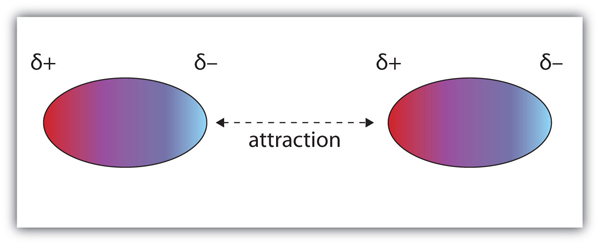
Attraction forces between molecules, arising due to a permanent or temporary dipole.

