7.1 Forms of Energy
Learning Objectives
- Define the law of conservation of matter and energy.
- Understand the different ways energy can be transformed into alternative forms.
- Define the study of thermochemistry and the types of energy of interest within physical chemistry.

When thinking about energy, your mind may naturally go to electricity – and why shouldn’t it! Electrical energy powers modern society (literally!). But where does it come from? The law of conservation of matter and energy states that neither can be created nor destroyed – it can only be transformed. If that’s the case, how is electrical energy generated?
Energy Transformations
Let’s take the example of a coal-fired power plant. To produce energy, the power plant needs to generate an electromotive force through the spinning of steam turbines. These turbines rotate through the use of pressurised steam, which is produced by raising the temperature of water until it boils. This water needs heat in order to undergo a change in its state of matter – supplied by burning coal through combustion (see Figure 7.1.1).
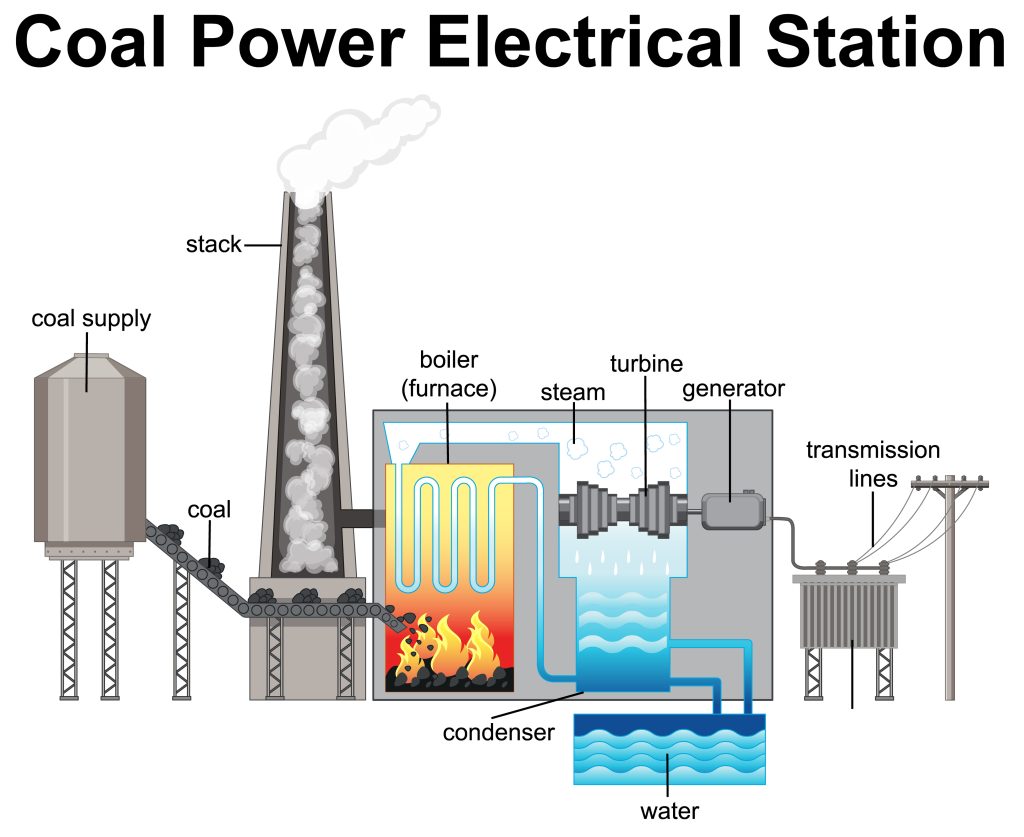
Consider the energy transformations that occur during this process: electrical energy is generated through the mechanical energy of the steam turbine, which is energy provided by the energy of the pressurised steam. The steam was generated by supplying thermal energy to the water to allow the molecules to overcome intermolecular forces and form a gas. The thermal energy was provided by harnessing the coal’s potential chemical energy through a reaction. Energy transformations are continuously occurring in our everyday lives, from cooking and eating to the nuclear fusion that occurs in the sun.
We can even take this one step further. The coal found in the Earth’s crust was generated through the intense heat and compression of carbon-based lifeforms, such as plants. For millions of years, those plants received light energy from the sun. Coal originally gets some of its energy from the sun! (Although it might not be renewable like others).
Energy transformations are all around us! Have a look at this interactive simulation to see the different types of energy transfers that can happen in daily processes. (Be sure to tick the ‘energy symbols’ box before you start.)
Simulation by PhET Interactive Simulations, University of Colorado Boulder, licensed under CC-BY-4.0 (https://phet.colorado.edu).
Forms of Energy
So, what exactly is energy? Energy is the ability to do work and is typically located within matter [1]. Work is performed against a force, such as gravity or electrical resistance. Here are some forms which energy can take:
| Kinetic Energy | Energy found within movement of particles and objects. | A car moving at speed. |
| Gravitational Potential Energy | Potential energy within objects that are positioned against a gravitational field. | A ball held upwards into the sky. |
| Chemical Energy | Energy harnessed through the rearrangement of atoms and molecules. | Covalent and Ionic Bonds |
| Light Energy | Energy found within photons and electromagnetic waves. | Infrared radiation from the sun. |
| Sound Energy | Energy associated with sound and air pressure. | The soundwaves produced from a speaker. |
| Thermal Energy | The heat energy found within hot objects. | The sensation of heat from holding a hot cup. |
| Nuclear Energy | Energy harnessed through the rearrangement of protons and neutrons. | The nuclear fission of uranium. |

In chemistry, we are mainly concerned with the interconversion of chemical energy and thermal energy. This field is specifically known as [pb_glossaryid=”663″]thermochemistry[/pb_glossary].
The available energy from seemingly unreactive sources is tremendous when considering all of the subatomic particles that compose matter (see Figure 7.1.2). The ways in which we harness that energy are dependent on the substances. We’ve already seen how combustion reactions can be utilised, but a variety of other reactions, such as redox, neutralisation and a wide variety of organic substitutions and reactions can also produce thermal energy.
As you will soon discover, heat plays an essential role in determining the extent and rates of reactions. It also is a major factor in chemical decomposition and other physical properties. Chemicals with a low flashpoint may ignite unexpectedly in experiments that involve temperature increase! As such, the management of energy is essential for any aspiring chemist.
Key Takeaways
- According to the law of conservation of energy and matter; energy can only be transformed, never created.
- Common forms of energy include kinetic, gravitational, chemical, light, sound, thermal and nuclear.
- Thermochemistry studies the interconversion of chemical and thermal energy.
Media Attributions
- Electricity transmission towers with glowing wires against blue sky – Energy concept © peterschreiber.media - stock.adobe.com is licensed under a All Rights Reserved license
- Diagram showing Coal Power Electrical Station © blueringmedia - stock.adobe.com is licensed under a All Rights Reserved license
- Metallic sodium in water © Alexandre - stock.adobe.com is licensed under a All Rights Reserved license
- ; photons are a notable exemption ↵
Law of physics that states that the total energy of an isolated system does not increase or decrease.
A machine that converts the rotational energy of a fluid into usable energy or work.
Attraction forces between molecules, arising due to a permanent or temporary dipole.
The ability to apply energy against a force.
Reduction-oxidation reaction.
The temperature needed for a liquid to generate sufficient vapour to combust.

