4.3 Classes of Chemical Reactions: Single and Double Displacement Reactions
Learning Objectives
- Recognise chemical reactions as single-replacement reactions and double-replacement reactions.
- Use the periodic table, an activity series, or solubility rules to predict whether single-replacement reactions or double-replacement reactions will occur.
Up to now, we have presented chemical reactions as a topic, but we have not discussed how the products of a chemical reaction can be predicted. Here, we will begin our study of certain types of chemical reactions that allow us to predict what the products of the reaction will be.
Single-Replacement Reactions
A single-replacement reaction is a chemical reaction in which one element is substituted for another element in a compound, generating a new element and a new compound as products. For example:
[latex]\ce{2HCl}{(aq)} + \ce{Zn}{(s)} → \ce{ZnCl}_{2}{(aq)} + \ce{H}_{2}{(g)}[/latex]
This is an example of a single-replacement reaction. The hydrogen atoms in [latex]\ce{HCl}[/latex] are replaced by [latex]\ce{Zn}[/latex] atoms, and in the process, a new element—hydrogen—is formed. Another example of a single-replacement reaction is:
[latex]\ce{2NaCl}{(aq)} + \ce{F}_{2}{(g)} → \ce{2NaF}{(s)} + \ce{Cl}_{2}{(g)}[/latex]
Here, the negatively charged ion changes from chloride to fluoride. A typical characteristic of a single-replacement reaction is that there is one element as a reactant and another element as a product.
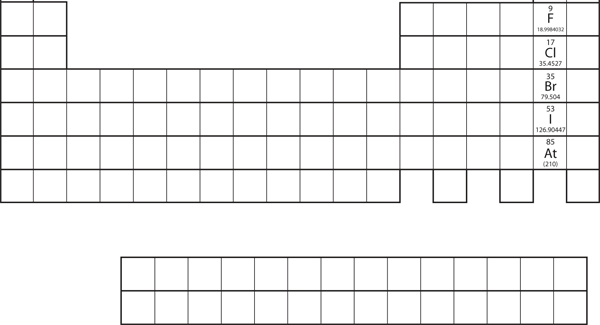
Not all proposed single-replacement reactions will occur between two given reactants. This is most easily demonstrated with fluorine, chlorine, bromine, and iodine. Collectively, these elements are called halogens and are in the next-to-last column on the periodic table (see Figure 4.3.1 “Halogens on the Periodic Table”). The elements on top of the column will replace the elements below them on the periodic table, but not the other way around. Thus, the reaction represented by:
[latex]\ce{CaI}_{2}{(s)} + \ce{Cl}_{2}{(g)} → \ce{CaCl}_{2}{(s)} + \ce{I}_{2}{(s)}[/latex]
This reaction will occur, but the reaction,
[latex]\ce{CaF}_{2}{(s)} + \ce{Br}_{2}{(ℓ)} → \ce{CaBr}_{2}{(s)} + \ce{F}_{2}{(g)}[/latex]
will not because bromine is below fluorine on the periodic table. This is just one of many ways the periodic table helps us understand chemistry.
Example 4.3.1
Problems
Will a single-replacement reaction occur? If so, identify the products.
- [latex]\ce{MgCl}_{2} + \ce{I}_{2} → ?[/latex]
- [latex]\ce{CaBr}_{2} + \ce{F}_{2} → ?[/latex]
Solutions
- Because iodine is below chlorine on the periodic table, a single-replacement reaction will not occur.
- Because fluorine is above bromine on the periodic table, a single-replacement reaction will occur, and the products of the reaction will be [latex]\ce{CaF}_{2}[/latex] and [latex]\ce{Br}_{2}[/latex].
Test Yourself
Will a single-replacement reaction occur? If so, identify the products.
[latex]\ce{FeI}_{2} + \ce{Cl}_{2} → ?[/latex]
Answer
Yes; [latex]\ce{FeCl}_{2}[/latex] and [latex]\ce{I}_{2}[/latex]
Chemical reactivity trends are easy to predict when replacing anions in simple ionic compounds—simply use their relative positions on the periodic table. However, when replacing the cations, the trends are not as straightforward. This is partly because there are so many elements that can form cations; an element in one column on the periodic table may replace another element nearby, or it may not. A list called the activity series does the same thing the periodic table does for halogens: it lists the elements that will replace other elements below them in single-replacement reactions. A simple activity series is shown below:
Activity Series for Cation Replacement in Single-Replacement Reactions
- Li
- K
- Ba
- Sr
- Ca
- Na
- Mg
- Al
- Mn
- Zn
- Cr
- Fe
- Ni
- Sn
- Pb
- H2
- Cu
- Hg
- Ag
- Pd
- Pt
- Au
Using an activity series is similar to using the positions of the halogens on the periodic table. An element on top will replace an element below it in compounds undergoing a single-replacement reaction. Elements will not replace elements above them in compounds.
Example 4.3.2
Problems
Use the activity series to predict the products, if any, of each equation.
- [latex]\ce{FeCl}_{2} + \ce{Zn} → ?[/latex]
- [latex]\ce{HNO}_{3} + \ce{Au} → ?[/latex]
Solutions
- Because zinc is above iron in the activity series, it will replace iron in the compound. The products of this single-replacement reaction are [latex]\ce{ZnCl}_{2}[/latex] and [latex]\ce{Fe}[/latex].
- Gold is below hydrogen in the activity series. As such, it will not replace hydrogen in a compound with the nitrate ion. No reaction is predicted.
Test Yourself
Use the activity series to predict the products, if any, of this equation.
[latex]\ce{AlPO}_{4} + \ce{Mg} → ?[/latex]
Answer
[latex]\ce{Mg}_{3}\ce{(PO_{4})}_{2}[/latex] and [latex]\ce{Al}[/latex]
Double-Replacement Reactions
A double-replacement reaction occurs when parts of two ionic compounds are exchanged, making two new compounds. A characteristic of a double-replacement equation is that there are two compounds as reactants and two different compounds as products. An example is:
[latex]\ce{CuCl}_{2}{(aq)} + \ce{2AgNO}_{3}{(aq)} → \ce{Cu(NO_{3})}_{2}{(aq)} + \ce{2AgCl}{(s)}[/latex]
There are two equivalent ways of considering a double-replacement equation: either the cations are swapped, or the anions are swapped. You cannot swap both; you would end up with the same substances you started with. Either perspective should allow you to predict the proper products as long as you pair a cation with an anion and not a cation with a cation or an anion with an anion.
Example 4.3.3
Problem
Predict the products of this double-replacement equation: [latex]\ce{BaCl}_{2} + \ce{Na}_{2}\ce{SO}_{4} → ?[/latex]
Solution
Thinking about the reaction as either switching the cations or switching the anions, we would expect the products to be [latex]\ce{BaSO}_{4}[/latex] and [latex]\ce{NaCl}[/latex].
Test Yourself
Predict the products of this double-replacement equation: [latex]\ce{KBr} + \ce{AgNO}_{3} → ?[/latex]
Answer
[latex]\ce{KNO}_{3}[/latex] and [latex]\ce{AgBr}[/latex]
Precipitation Reactions and Solubility Rules
Predicting whether a double-replacement reaction will occur is somewhat more difficult than predicting a single-replacement reaction. However, there is one type of double-replacement reaction that we can predict: the precipitation reaction. A precipitation reaction occurs when two ionic compounds are dissolved in water and form a new ionic compound that does not dissolve; this new compound falls out of the solution as a solid precipitate. The formation of a solid precipitate is the driving force that makes the reaction proceed.
To judge whether double-replacement reactions will occur, we need to know what kinds of ionic compounds form precipitates. For this, we use solubility rules, which are general statements that predict which ionic compounds dissolve (are soluble) and which do not (are not soluble or insoluble). Tables 4.3.1 and 4.3.2 list some general solubility rules. These rules highlight that we need to consider each ionic compound (both the reactants and the possible products) in light of the solubility rules in Tables 4.3.1 and 4.3.2. If a compound is soluble, we use the (aq) label with it, indicating that it dissolves. If a compound is not soluble, we use the (s) label with it and assume that it will precipitate out of the solution. If all possible products are soluble in a reaction, then no precipitation reaction will be expected.
| These compounds generally dissolve in water (are soluble): | Exceptions: |
|---|---|
| All compounds of Li+, Na+, K+, Rb+, Cs+, and NH4+ | None |
| All compounds of NO3− and C2H3O2− | None |
| Compounds of Cl−, Br−, I− | Ag+, Hg22+, Pb2+ |
| Compounds of SO42 | Hg22+, Pb2+, Sr2+, Ba2+ |
| These compounds generally do not dissolve in water (are insoluble): | Exceptions: |
|---|---|
| Compounds of CO32− and PO43− | Compounds of Li+, Na+, K+, Rb+, Cs+, and NH4+ |
| Compounds of OH− | Compounds of Li+, Na+, K+, Rb+, Cs+, NH4+, Sr2+, and Ba2+ |
To illustrate the rules mentioned in the previous paragraph:
- consider the possible double-replacement reaction between [latex]\ce{Na}_{2}\ce{SO}_{4}[/latex] and [latex]\ce{SrCl}_{2}[/latex]. The solubility rules say that all ionic sodium compounds are soluble, and all ionic chloride compounds are soluble except for [latex]\ce{Ag}^{+}[/latex], [latex]\ce{Hg}^{2+}[/latex], and [latex]\ce{Pb}^{2+}[/latex], which are not being considered here. Therefore, [latex]\ce{Na}_{2}\ce{SO}_{4}[/latex] and [latex]\ce{SrCl}_{2}[/latex] are both soluble.
- The possible double-replacement reaction products are [latex]\ce{NaCl}[/latex] and [latex]\ce{SrSO}_{4}[/latex]. Are these soluble? [latex]\ce{NaCl}[/latex] is (by the same rule we just quoted), but what about [latex]\ce{SrSO}_{4}[/latex]? Compounds of the sulphate ion are generally soluble, but [latex]\ce{Sr}^{2+}[/latex] is an exception: we expect it to be insoluble—a precipitate. Therefore, we expect a reaction to occur, and the balanced chemical equation would be:
[latex]\ce{Na}_{2}\ce{SO}_{4}{(aq)} + \ce{SrCl}_{2}{(aq)} → \ce{2NaCl}{(aq)} + \ce{SrSO}_{4}{(s)}[/latex]
- You would expect to see a visual change corresponding to [latex]\ce{SrSO}_{4}[/latex]precipitating out of solution (Figure 4.3.2 “Double-Replacement Reactions”).

Example 4.3.4
Problems
Will a double-replacement reaction occur? If so, identify the products.
- [latex]\ce{Ca(NO{3})}_{2} + \ce{KBr} → ?[/latex]
- [latex]\ce{NaOH} + \ce{FeCl}_{2} → ?[/latex]
Solutions
- According to the solubility rules, both [latex]\ce{Ca(NO_{3})}_{2}[/latex] and [latex]\ce{KBr}[/latex] are soluble. Now, we consider what the double-replacement products would be by switching the cations (or the anions)—namely, [latex]\ce{CaBr}_{2}[/latex] and [latex]\ce{KNO}_{3}[/latex]. However, the solubility rules predict that these two substances would also be soluble so that no precipitate would form. Thus, we predict no reaction in this case.
- According to the solubility rules, both [latex]\ce{NaOH}[/latex] and [latex]\ce{FeCl}_{2}[/latex] are expected to be soluble. If we assume that a double-replacement reaction may occur, we need to consider the possible products, which would be [latex]\ce{NaCl}[/latex] and [latex]\ce{Fe(OH)}_{2}[/latex]. [latex]\ce{NaCl}[/latex] is soluble, but according to the solubility rules, [latex]\ce{Fe(OH)}_{2}[/latex] is not. Therefore, a reaction would occur, and [latex]\ce{Fe(OH)}_{2}{(s)}[/latex] would precipitate out of the solution. The balanced chemical equation is: [latex]\ce{2NaOH}{(aq)} + \ce{FeCl}_{2}{(aq)} → \ce{2NaCl}{(aq)} + \ce{Fe(OH)}_{2}{(s)}[/latex]
Test Yourself
Will a double-replacement equation occur? If so, identify the products.
[latex]\ce{Sr(NO_{3})}_{2} + \ce{KCl} → ?[/latex]
Answer
No reaction; all possible products are soluble.
Key Takeaways
- A single-replacement reaction replaces one element for another in a compound.
- The periodic table or an activity series can help predict whether a single-replacement reaction will occur.
- A double-replacement reaction exchanges the cations (or the anions) of two ionic compounds.
- A precipitation reaction is a double-replacement reaction in which one product is a solid precipitate.
- Solubility rules are used to predict whether some double-replacement reactions will occur.
Exercises
Media Attributions
Halogens are a group of elements in the periodic table that belong to Group 17. The halogen group includes fluorine (F), chlorine (Cl), bromine (Br), iodine (I) etc.

