3.2 Ionic Bonds and Electronegativity
Learning Objectives
- Know how ions form.
- Learn the characteristic charges that ions have.
- Construct a proper formula for an ionic compound.
- Generate a proper name for an ionic compound.
So far, we have discussed elements and compounds that are electrically neutral. However, this is not always the case. As we have seen within Lewis Dot Diagrams, electrons can move from one atom to another; when they do, species with overall electric charges are formed. Such species are called ions. Species with overall positive charges are termed cations, while species with overall negative charges are called anions (seen in Table 3.2.1). Remember, ions are formed only when electrons move from one atom to another; a proton never moves from one atom to another. Compounds formed from positive and negative ions are called ionic compounds.
| Ions formed by losing a single electron | H+
Na+ K+ Rb+ Ag+ Au+ |
Ions formed by gaining a single electron | F−
Cl− Br− I− |
| Ions formed by losing two electrons | Mg2+
Ca2+ Sr2+ Fe2+ Co2+ Ni2+ Cu2+ Zn2+ Sn2+ Hg2+ Pb2+ |
Ions formed by gaining two electrons | O2−
S2− Se2− |
| Ions formed by losing three electrons | Sc3+
Fe3+ Co3+ Ni3+ Au3+ Al3+ Cr3+ |
Ions formed by gaining three electrons | N3−
P3− |
| Ions formed by losing four electrons | Ti4+
Sn4+ Pb4+ |
As noted in the table, some elements will appear multiple times. For instance, all metals can form more than one possible charge. For example, iron atoms can form 2+ cations or 3+ cations. Cobalt is another element that can form more than one possible charged ion (2+ and 3+), while lead can form 2+ or 4+ cations. Unfortunately, there is little understanding of which two charges a metal atom may take, so it is best to just memorise the possible charges a particular element can have.
Note the convention for indicating an ion. The magnitude of the charge is listed as a right superscript next to the symbol of the element. If the charge is a single positive or negative one, the number 1 is not written; if the magnitude of the charge is greater than 1, then the number is written before the + or − sign. An element symbol without a charge written next to it is assumed to be the uncharged atom.
Naming Ions
Main group cations are named using the name of the element followed by ion. For instance, [latex]\ce{Li}^{+}[/latex]: Lithium ion, [latex]\ce{Mg}^{2+}[/latex]: Magnesium ion and [latex]\ce{Al}^{3+}[/latex]: Aluminium ion.
Transition metals ([latex]d, f[/latex] block) and some [latex]p[/latex] block elements can form multiple cations such as [latex]\ce{Cu}^{+}[/latex] and [latex]\ce{Cu}^{2+}[/latex], [latex]\ce{Pb}^{2+}[/latex] and [latex]\ce{Pb}^{4+}[/latex]. These types of cations can be named in two ways. The first is that the ion with the smaller charge ends with “-ous” and the larger charge ends with “-ic”. For instance, [latex]\ce{Cu}^{+}[/latex]is cuprous ion and [latex]\ce{Cu}^{2+}[/latex] is cupric ion. The more common way is writing the charge of the ion in Roman numerals within parenthesis next to the element name. For example, [latex]\ce{Cu}^{+}[/latex]is Copper[latex]\left(\mathrm{I}\right)[/latex] ion, and [latex]\ce{Cu}^{2+}[/latex] is Copper[latex]\left(\mathrm{II}\right)[/latex] ion.
Anions are named by removing the ending of the element name and replacing it with “-ide”. For instance, the anion formed by [latex]\ce{Cl}[/latex] is known as a chloride ion.
Polyatomic ions consist of more than one atom. For example, [latex]\ce{SO}_{4}^{2-}[/latex] (sulphate), [latex]\ce{NO}_{3}^{-}[/latex] (nitrate), [latex]\ce{PO}_{4}^{3-}[/latex] (phosphate) and [latex]\ce{NH}_{4}^{+}[/latex] (ammonium) ions. Atoms in polyatomic ions are held together by covalent bonds. These generally have unique names as seen in Table 3.2.3.
| Name | Formula and Charge |
|---|---|
| ammonium | NH4+ |
| acetate | C2H3O2−, or CH3COO− |
| bicarbonate (hydrogen carbonate) | HCO3− |
| bisulphate (hydrogen sulphate) | HSO4− |
| carbonate | CO32− |
| chlorate | ClO3− |
| chromate | CrO42− |
| cyanide | CN− |
| dichromate | Cr2O72− |
| hydroxide | OH− |
| nitrate | NO3− |
| nitrite | NO2− |
| peroxide | O22− |
| perchlorate | ClO4− |
| permanganate | MnO4– |
| phosphate | PO43− |
| sulphate | SO42− |
| sulphite | SO32− |
| triiodide | I3− |
Ionic bonds

Ionic bonds are formed by the complete transfer of electrons from one atom to another. Ionic bonds are generally created between metals and nonmetals. Metals provide the cation (lose electrons), and nonmetals (gain electrons) provide the anion. For an ionic bond to form, there must be a large difference in electronegativity between two elements.
Electronegativity is the power of an atom in a molecule to attract electrons. If you recall from 2.4 Periodic Trends, electronegativity becomes higher as one moves from the bottom left of the periodic table to the halogens. As a result, bonds between elements from the far left and far right of the periodic table are typically ionic. For an ionic compound to be formed, the difference between the electronegativities of two participating atoms must be larger than or equal to two.
Please note that, unlike other measurements, electronegativity is a unitless quantity. It is not measured in a unit such as grams or metres — it is simply a number. See Figure 3.2.1 for the electronegativities of the main group elements.
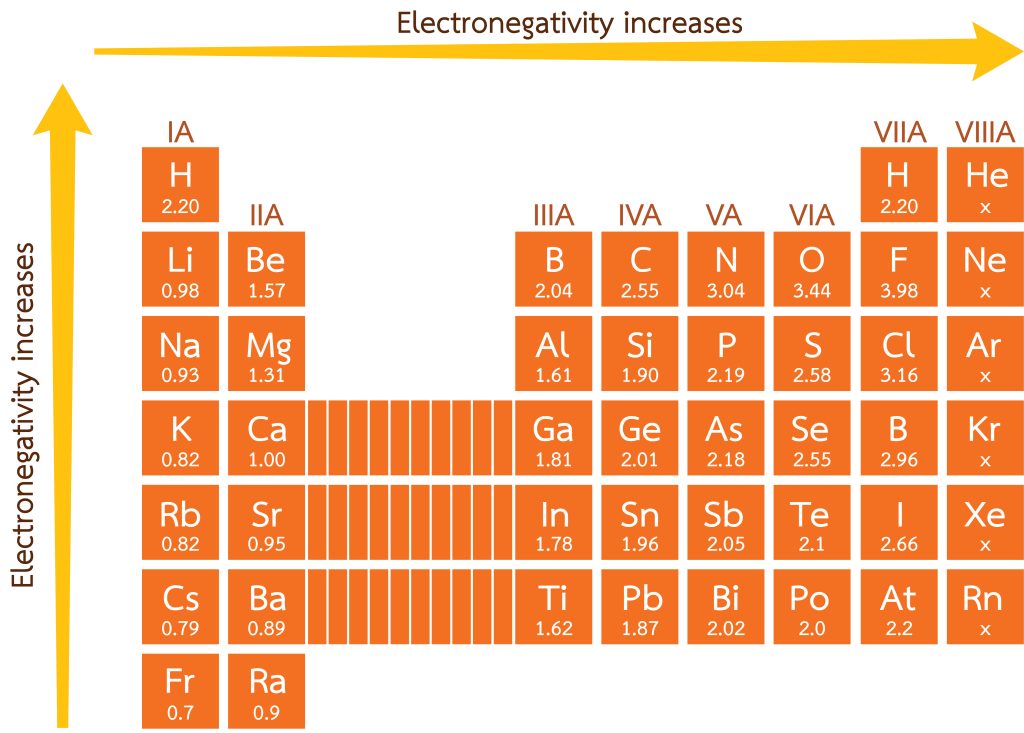
As an example, for the commonly-known ionic compound, sodium chloride ([latex]\ce{NaCl}[/latex]), the difference in electronegativity of [latex]\ce{Na}[/latex] (EN[latex]=0.93[/latex]) and [latex]\ce{Cl}[/latex] (EN[latex]=3.16[/latex]) is [latex]2.23[/latex]. The larger difference in electronegativity creates electrostatic interactions between the electrons of one atom and the nucleus of the other atom. Ionic compounds are held together by electrostatic interactions between the cations and anions involved.
Names and Formulas for Ionic Compounds
Chemical formulas for ionic compounds are called ionic formulas. A proper ionic formula has a cation and an anion in it; an ionic compound is never formed between two cations only or two anions only. The key to writing proper ionic formulas is to ensure the total positive charge balances out the total negative charge. Consider the ionic compound between [latex]\ce{Na}^{+}[/latex] and [latex]\ce{Cl}^{-}[/latex]. Each ion has a single charge (one positive and one negative), so we need only one ion of each to balance the overall charge. When writing the ionic formula, we follow two additional conventions: (1) write the formula for the cation first and the formula for the anion next, but (2) do not write the charges on the ions. Thus, for the compound between [latex]\ce{Na}^{+}[/latex] and [latex]\ce{Cl}^{-}[/latex], we have the ionic formula [latex]\ce{NaCl}[/latex].

Because the charges on the ions are constant, sometimes we have to have more than one cation or an anion to balance the overall positive and negative charges. For the ionic compound between [latex]\ce{Mg}^{2+}[/latex] ions and [latex]\ce{Cl}^{-}[/latex] ions, we now consider the fact that the charges have different magnitudes; 2+ on the magnesium ion and 1− on the chloride ion. To balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on the one magnesium ion. Rather than write the formula MgClCl, we combine two chloride ions and write it with a 2 subscript: [latex]\ce{MgCl}_{2}[/latex].
Naming ionic compounds requires you to combine the name of the cation and the name of the anion, in both cases omitting the word ion. Do not use numerical prefixes if more than one ion is necessary to balance the charges. [latex]\ce{NaCl}[/latex] is sodium chloride, a combination of the name of the cation (sodium) and the anion (chloride). [latex]\ce{MgO}[/latex] is magnesium oxide. [latex]\ce{MgCl}_{2}[/latex] is magnesium chloride — not magnesium dichloride.
In naming ionic compounds whose cations can have more than one possible charge, we must also include the charge, in parentheses and in roman numerals, as part of the name. Hence [latex]\ce{FeS}[/latex] is iron(II) sulphide, while [latex]\ce{Fe}_2{S}_3[/latex] is iron(III) sulphide. No numerical prefixes appear in the name. The number of ions in the formula is dictated by the need to balance the positive and negative charges.
There are two ways of writing chemical formulas.
Method 1:
First, write down the symbol of the cation with its charge, followed by the anion.
[latex]\ce{Ca}^{2+}\ce{F}^{-}[/latex]
Next, cross each ion’s charge (only the numerical value, not the sign) and write these numbers down as subscripts. So, the calcium ion’s charge will be written next to the fluoride ion, and the fluoride ion’s charge will be written next to the calcium ion, in both cases as subscripts:
\[\begin{align*}\ce{Ca}^{2+} & \searrow\swarrow\ce{F}^{-}\\& \ce{CaF}_{2}\end{align*}\]
Method 2:
[latex]\ce{Ca}[/latex] ion has a [latex]+2[/latex] charge, and [latex]\ce{F}[/latex] ion has a [latex]-1[/latex] charge. Two [latex]\ce{F}[/latex] ions, each with [latex]-1[/latex] charge, will neutralise the [latex]+2[/latex] charge of [latex]\ce{Ca}[/latex] ion. Therefore, the chemical formula is [latex]\ce{CaF2}[/latex]
Physical Properties of Ionic Compounds

Your favourite energy sports drinks (see Figure 3.2.3) typically love to advertise the number of electrolytes they have; but what exactly are they? When one exercises or is sick, the sweat excreted causes the body to lose essential ions important for carrying electrical signals. Ions such as [latex]\ce{Na}^{+}[/latex], [latex]\ce{K}^{+}[/latex] and [latex]\ce{Cl}^{-}[/latex] have to be continuously replaced. Sports drinks and rehydration solutions contain increased amounts of these ions to ensure one retains a healthy number of conductive ions.
Ionic compounds are typically crystalline solids (like table salt) with high melting and boiling points that are soluble in polar solvents (such as water). When in their aqueous state, ionic compounds are able to conduct electricity through the movement of ions.
Key Takeaways
- Ions form when species lose or gain electrons, commonly when compounds dissolve in water.
- Metal ions have a variety of oxidation states, and can form numerous different ions.
- Cations refer to positively charged ions, while anions refer to negatively charged.
- When the difference in electronegativity is 2 or more, an ionic bond forms between two atoms.
- The main group cations are named using the name of the element followed by an ion, while anions feature the suffix “-ide.”
- Many polyatomic ions exist that are commonly referred to as ammonium, cyanide, and hydroxide.
- Ions in solution are conductors of electricity, an important property for healthy living within living beings.
Exercises
Media Attributions
A group of chemical elements, compounds or molecules with similar characteristics.
A species with an overall electric charge.
A species with an overall positive charge.
A species with an overall negative charge.
The s-block and p-block elements.
A chemical bond formed by two atoms sharing electrons.
Power of an atom to attract electrons; determines bond formation.
A molecule or bond that experiences a (partial) charge.
Dissolved in or solvated by water.

