6.1 Definitions and Theories of Acids and Bases
Learning Objectives
- Identify an Arrhenius acid and an Arrhenius base.
- Write the chemical reaction between an Arrhenius acid and an Arrhenius base.
- Identify a Brønsted-Lowry acid and a Brønsted-Lowry base.
- Identify conjugate acid-base pairs in an acid-base reaction.
Video 6.1.1: Conjugate Acid-Base Pairs. Explanation of conjugate acid-base pairs in chemistry. Video attribution: “Conjugate Acid-Base Pairs” by RMIT Library. © 2024 RMIT Library, licensed under CC BY-NC-SA 4.0.
Arrhenius Acids and Bases
Historically, the first chemical definition of an acid and a base was put forward by Svante Arrhenius, a Swedish chemist, in 1884. An Arrhenius acid is a compound that increases the [latex]\ce{H^{+}}[/latex] ion concentration in aqueous solution. The [latex]\ce{H^{+}}[/latex] ion is just a bare proton, and it is rather clear that bare protons are not floating around in an aqueous solution. Instead, chemistry has defined the hydronium ion [latex]\ce{H_{3}O^{+}}[/latex] as the actual chemical species that represents an [latex]\ce{H^{+}}[/latex]ion. [latex]\ce{H^{+}}[/latex] ions and [latex]\ce{H_{3}O^{+}}[/latex] ions are often considered interchangeable when writing chemical equations (although a properly balanced chemical equation should also include the additional [latex]\ce{H_{2}O}[/latex]). Classic Arrhenius acids can be considered ionic compounds in which [latex]\ce{H^{+}}[/latex] is the cation. Table 6.1.1 lists examples of Arrhenius acids and their names.
| Formula | Name |
|---|---|
| [latex]\ce{HC_{2}H_{3}O_{2}}[/latex] (also written [latex]\ce{CH_{3}COOH}[/latex]) | acetic acid |
| [latex]\ce{HClO_{3}}[/latex] | chloric acid |
| [latex]\ce{HCl}[/latex] | hydrochloric acid |
| [latex]\ce{HBr}[/latex] | hydrobromic acid |
| [latex]\ce{HI}[/latex] | hydriodic acid |
| [latex]\ce{HF}[/latex] | hydrofluoric acid |
| [latex]\ce{HNO_{3}}[/latex] | nitric acid |
| [latex]\ce{H_{2}C_{2}O_{4}}[/latex] | oxalic acid |
| [latex]\ce{HClO_{4}}[/latex] | perchloric acid |
| [latex]\ce{H_{3}PO_{4}}[/latex] | phosphoric acid |
| [latex]\ce{H_{2}SO_{4}}[/latex] | sulfuric acid |
| [latex]\ce{H_{2}SO_{3}}[/latex] | sulfurous acid |
An Arrhenius base is a compound that increases the [latex]\ce{OH^{-}}[/latex] ion concentration in aqueous solution. Ionic compounds of the [latex]\ce{OH^{-}}[/latex] ion are classic Arrhenius bases.
Example 6.1.1
Problem
Identify each compound as an Arrhenius acid, an Arrhenius base, or neither.
- [latex]\ce{HNO_{3}}[/latex]
- [latex]\ce{CH_{3}OH}[/latex]
- [latex]\ce{Mg(OH)_{2}}[/latex]
Solution
- This compound is an ionic compound between [latex]\ce{H^{+}}[/latex]ions and [latex]\ce{NO_{3}^{-}}[/latex] ions, so it is an Arrhenius acid.
- Although this formula has an [latex]\ce{OH}[/latex] in it, we do not recognize the remaining part of the molecule as a cation. It is neither an acid nor a base. (In fact, it is the formula for methanol, an organic compound.)
- This formula also has an [latex]\ce{OH}[/latex] in it, but this time, we recognize that the magnesium is present as [latex]\ce{Mg^{2+}}[/latex] cations. As such, this is an ionic compound of the [latex]\ce{OH^{-}}[/latex] ion and is an Arrhenius base.
Test Yourself
Identify each compound as an Arrhenius acid, an Arrhenius base, or neither.
- [latex]\ce{KOH}[/latex]
- [latex]\ce{H_{2}SO_{4}}[/latex]
- [latex]\ce{C_{2}H_{6}}[/latex]
Answer
- Arrhenius base
- Arrhenius acid
- neither
Acids have some properties in common. They turn litmus, a plant extract, red. They react with some metals to give off [latex]\ce{H_{2}}[/latex] gas. They react with carbonate and hydrogen carbonate salts to give off [latex]\ce{CO_{2}}[/latex] gas. Acids that are ingested typically have a sour, sharp taste. (The name acid comes from the Latin word acidus, meaning “sour.”) Bases also have some properties in common. They are slippery to the touch, turn litmus blue, and have a bitter flavour if ingested.
Acids and bases have another property: they react with each other to make water and an ionic compound called a salt. A salt, in chemistry, is any ionic compound made by combining an acid with a base. A reaction between an acid and a base is called a neutralisation reaction and can be represented as follows:
[latex]\ce{acid} + \ce{base} → \ce{H_{2}O} + \ce{salt}[/latex]
The stoichiometry of the balanced chemical equation depends on the number of [latex]\ce{H^{+}}[/latex] ions in the acid and the number of [latex]\ce{OH^{-}}[/latex] ions in the base.
Brønsted-Lowry Acid-base Theory
The Arrhenius definition of acid and base is limited to aqueous (that is, water) solutions. Although this is useful because water is a common solvent, it is limited to the relationship between the [latex]\ce{H^{+}}[/latex] ion and the [latex]\ce{OH^{-}}[/latex] ion. What would be useful is a more general definition that would be more applicable to other chemical reactions and, importantly, independent of [latex]\ce{H_{2}O}[/latex].
In 1923, Danish chemist Johannes Brønsted and English chemist Thomas Lowry independently proposed new definitions for acids and bases, ones that focus on proton transfer. A Brønsted-Lowry acid is any species that can donate a proton ([latex]\ce{H^{+}}[/latex]) to another molecule. A Brønsted-Lowry base is any species that can accept a proton from another molecule. In short, a Brønsted-Lowry acid is a proton donor (PD), while a Brønsted-Lowry base is a proton acceptor (PA).
The Brønsted-Lowry definition covers the Arrhenius definition of acids and bases. Consider the prototypical Arrhenius acid-base reaction:
[latex]\begin{array}{ccccc} \ce{H+(aq)}&+&\ce{OH-(aq)}&\rightarrow&\ce{H2O(\ell)} \\ \\ \text{(acid)}&&\text{(base)}&& \end{array}[/latex]
The acid species and base species are marked. The proton, however, is (by definition) a proton donor (labelled PD), while the [latex]\ce{OH^{-}}[/latex] ion is acting as the proton acceptor (labelled PA):
[latex]\begin{array}{ccccc} \ce{H+(aq)}&+&\ce{OH-(aq)}&\rightarrow&\ce{H2O(\ell)} \\ \\ \text{(PD)}&&\text{(PA)}&& \end{array}[/latex]
The proton donor is a Brønsted-Lowry acid, and the proton acceptor is the Brønsted-Lowry base:
[latex]\begin{array}{ccccc} \ce{H+(aq)}&+&\ce{OH-(aq)}&\rightarrow&\ce{H2O(\ell)} \\ \\ \text{(BL acid)}&&\text{(BL base)}&& \end{array}[/latex]
Thus [latex]\ce{H^{+}}[/latex] is an acid by both definitions, and [latex]\ce{OH^{-}}[/latex] is a base by both definitions.
Ammonia ([latex]\ce{NH_{3}}[/latex]) is a base even though it does not contain [latex]\ce{OH^{-}}[/latex] ions in its formula. Instead, it generates [latex]\ce{OH^{-}}[/latex] ions as the product of a proton-transfer-reaction with [latex]\ce{H_{2}O}[/latex] molecules; [latex]\ce{NH_{3}}[/latex] acts like a Brønsted-Lowry base, and [latex]\ce{H_{2}O}[/latex] acts like a Brønsted-Lowry acid:
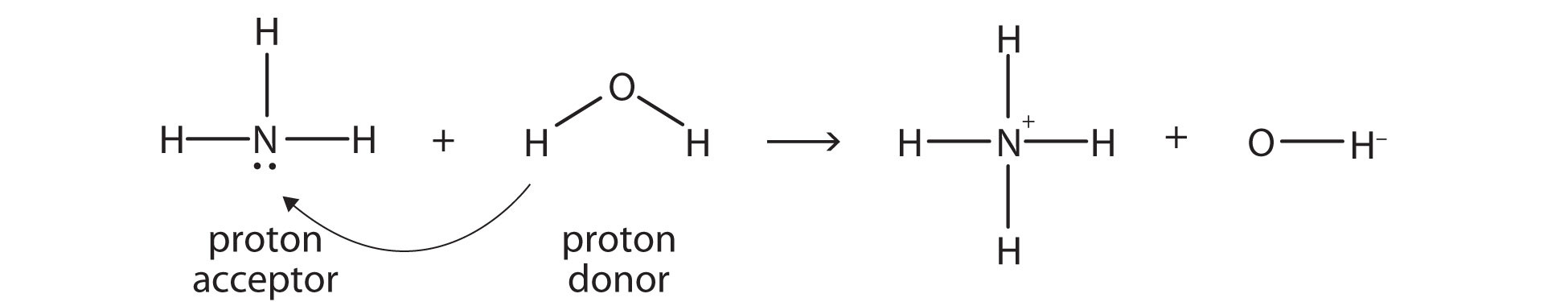
A reaction with water is called hydrolysis; we say that [latex]\ce{NH_{3}}[/latex] hydrolyses to make [latex]\ce{NH_{4}^{+}}[/latex] ions and [latex]\ce{OH^{-}}[/latex]ions, as shown above.
Even the dissolving of an Arrhenius acid in water can be considered a Brønsted-Lowry acid-base reaction. Consider the process of dissolving [latex]\ce{HCl}{(g)}[/latex] in water to make an aqueous solution of hydrochloric acid. The process can be written as follows:
[latex]\ce{HCl(g)} + \ce{H_{2}O}{(ℓ)} → \ce{H_{3}O}^{+}{(aq)} + \ce{Cl^{-}}{(aq)}[/latex]
[latex]\ce{HCl(g)}[/latex] is the proton donor and, therefore, a Brønsted-Lowry acid, while [latex]\ce{H_{2}O}[/latex] is the proton acceptor and a Brønsted-Lowry base. These two examples show that [latex]\ce{H_{2}O}[/latex] can act as both a proton donor and a proton acceptor, depending on what other substance is in the chemical reaction. A substance that can act as a proton donor or a proton acceptor is called amphiprotic. Water is probably the most common amphiprotic substance we will encounter, but other substances are also amphiprotic.
Examples 6.1.2
Problem
Identify the Brønsted-Lowry acid and the Brønsted-Lowry base in this chemical equation.
[latex]\ce{C_{6}H_{5}OH} + \ce{NH_{2}^{-}} → \ce{C_{6}H_{5}O}^{-} + \ce{NH_{3}}[/latex]
Solution
The [latex]\ce{C_{6}H_{5}OH}[/latex] molecule is losing an [latex]\ce{H^{+}}[/latex]; it is the proton donor and the Brønsted-Lowry acid. The [latex]\ce{NH_{2}^{-}}[/latex] ion (called the amide ion) is accepting the [latex]\ce{H^{+}}[/latex] ion to become [latex]\ce{NH_{3}}[/latex], so it is the Brønsted-Lowry base.
Test Yourself
Identify the Brønsted-Lowry acid and the Brønsted-Lowry base in this chemical equation.
[latex]\ce{Al(H_{2}O)_{6}^{3+}} + \ce{H_{2}O} → \ce{Al(H_{2}O)_{5}(OH)^{2+}} + \ce{H_{3}O^{+}}[/latex]
Answer
Brønsted-Lowry acid: [latex]\ce{Al(H_{2}O)_{6}^{3+}}[/latex]; Brønsted-Lowry base: [latex]\ce{H_{2}O}[/latex]
Observe the reaction between [latex]\ce{NH_{3}}[/latex] and [latex]\ce{H_{2}O}[/latex]:
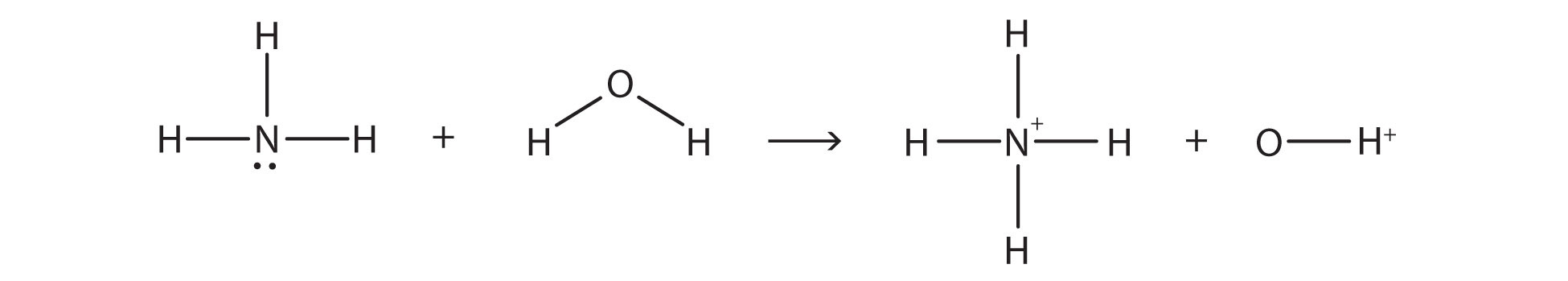
The chemical reaction does not go to completion; rather, the reverse process occurs as well, and eventually, the two processes cancel out any additional change. At this point, we say the chemical reaction is at equilibrium. Both processes still occur, but any net change by one process is countered by the same net change by the other process; it is a dynamic, rather than a static, equilibrium. Because both reactions are occurring, it makes sense to use a double arrow instead of a single arrow:
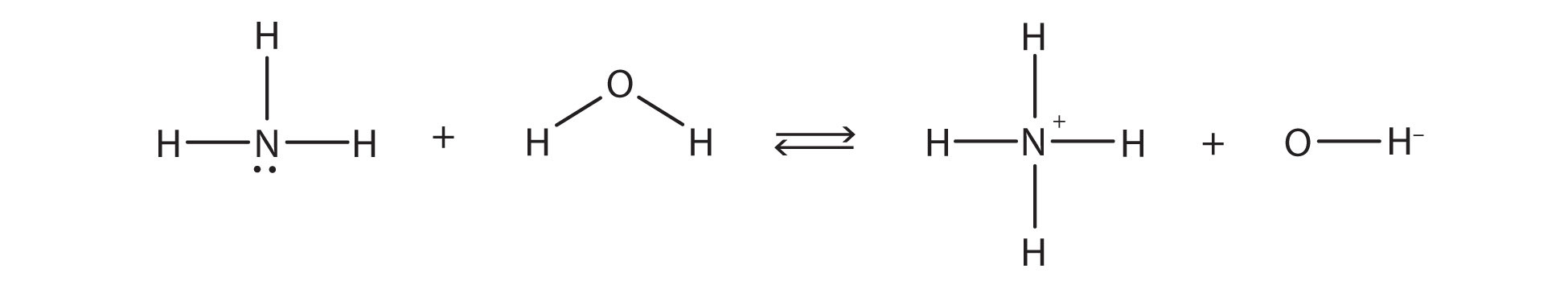
What do you notice about the reverse reaction? The [latex]\ce{NH_{4}^{+}}[/latex] ion is donating a proton to the [latex]\ce{OH^{-}}[/latex] ion, which is accepting it. This means that the [latex]\ce{NH_{4}^{+}}[/latex] ion is acting as the proton donor, or Brønsted-Lowry acid, while [latex]\ce{OH^{-}}[/latex] ion, the proton acceptor, is acting as a Brønsted-Lowry base. The reverse reaction is also a Brønsted-Lowry acid-base reaction:
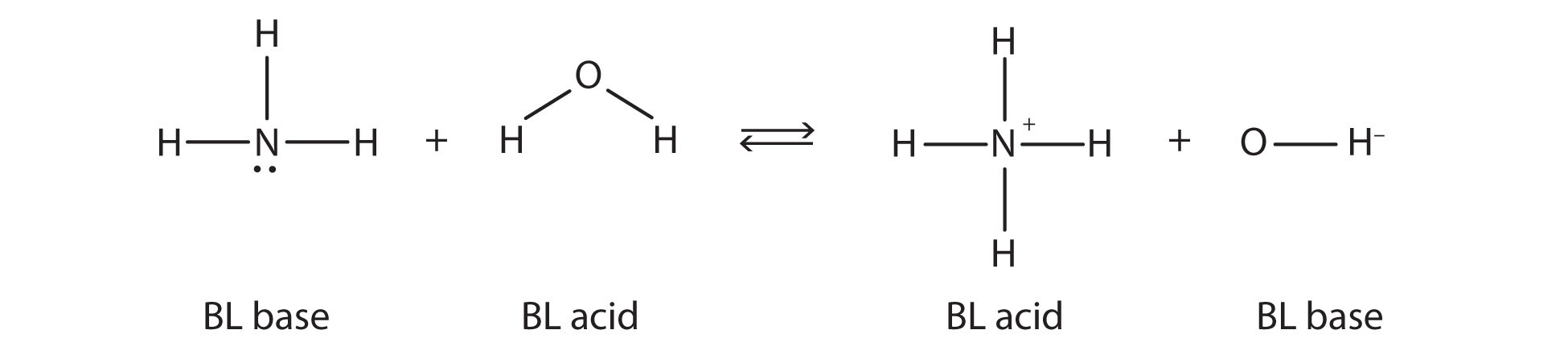
This means that both reactions are acid-base reactions by the Brønsted-Lowry definition. If you consider the species in this chemical reaction, two sets of similar species exist on both sides. Within each set, the two species differ by a proton in their formulas, and one member of the set is a Brønsted-Lowry acid, while the other member is a Brønsted-Lowry base. These sets are marked here:
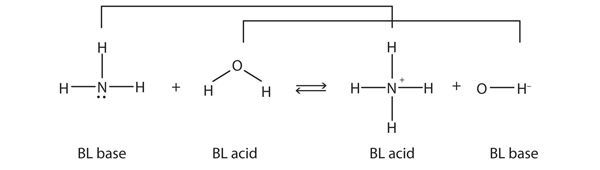
The two sets — [latex]\ce{NH_{3}}/\ce{NH_{4}^{+}}[/latex] and [latex]\ce{H{2}O}/\ce{OH^{-}}[/latex] — are called conjugate acid-base pairs. We say that [latex]\ce{NH_{4}^{+}}[/latex] is the conjugate acid of [latex]\ce{NH_{3}}, \ce{OH^{-}}[/latex] is the conjugate base of [latex]\ce{H_{2}O}[/latex], and so forth. Every Brønsted-Lowry acid-base reaction can be labelled with two conjugate acid-base pairs.
Examples 6.1.3
Problem
Identify the conjugate acid-base pairs in this equilibrium.
[latex]\ce{(CH_{3})_{3}N} + \ce{H_{2}O} ⇄ \ce{(CH_{3})_{3}NH^{+}} + \ce {OH^{-}}[/latex]
Solution
One pair is[latex]\ce{H_{2}O}[/latex] and [latex]\ce{OH^{-}}[/latex], where [latex]\ce{H_{2}O}[/latex] has one more [latex]\ce{H^{+}}[/latex] and is the conjugate acid, while [latex]\ce{OH^{-}}[/latex] has one less [latex]\ce{H^{+}}[/latex] and is the conjugate base. The other pair consists of [latex]\ce{(CH_{3})_{3}N}[/latex] and [latex]\ce{(CH_{3})_{3}NH^{+}}[/latex], where [latex]\ce{(CH_{3})_{3}NH^{+}}[/latex] is the conjugate acid (it has an additional proton) and [latex]\ce{(CH_{3})_{3}N}[/latex] is the conjugate base.
Test Yourself
Identify the conjugate acid-base pairs in this equilibrium.
[latex]\ce{NH_{2}^{+}}+ \ce{H_{2}O} ⇄ \ce{NH_{3}} + \ce{OH^{-}}[/latex]
Answer
[latex]\ce{H_{2}O}[/latex] (acid) and[latex]\ce{OH^{-}}[/latex] (base); [latex]\ce{NH_{2}^{+}}[/latex] (base) and [latex]\ce{NH_{3}}[/latex] (acid)
Household Acids and Bases
Many household products are acids or bases. For example, the owner of a swimming pool may use muriatic acid to clean the pool. Muriatic acid is another name for [latex]\ce{HCl}{(aq)}[/latex]. Vinegar is a dilute solution of acetic acid [latex]\ce{HC_{2}H_{3}O_{2}}{(aq)}[/latex] In a medicine chest, one may find a bottle of vitamin C tablets; the chemical name of vitamin C is ascorbic acid ([latex]\ce{HC_{6}H_{7}O_{6}}[/latex]).

One of the more familiar household bases is [latex]\ce{NH_{3}}[/latex], which is found in numerous cleaning products. [latex]\ce{NH_{3}}[/latex] is a base because it increases the [latex]\ce{OH^{-}}[/latex] ion concentration by reacting with [latex]\ce{H_{2}O}[/latex]:
[latex]\ce{NH_{3}}{(aq)} + \ce{H_{2}O}{(ℓ)} → \ce{NH_{4}^{+}}{(aq)} + \ce{OH^{-}}{(aq)}[/latex]
Many soaps are also slightly basic because they contain compounds that act as Brønsted-Lowry bases, accepting protons from [latex]\ce{H_{2}O}[/latex] and forming excess [latex]\ce{OH^{-}}[/latex] ions. This is one explanation for why soap solutions are slippery.
Perhaps the most dangerous household chemical is the lye-based drain cleaner. Lye is a common name for [latex]\ce{NaOH}[/latex], although it is also used as a synonym for [latex]\ce{KOH}[/latex]. Lye is an extremely caustic chemical that can react with grease, hair, food particles, and other substances that may build up and clog a water pipe.
Watch the following simulation about acids and bases.
Key Takeaways
- An Arrhenius acid is a compound that increases the [latex]\ce{H^{+}}[/latex] ion concentration in aqueous solution.
- An Arrhenius base is a compound that increases the [latex]\ce{OH^{-}}[/latex] ion concentration in aqueous solution.
- The reaction between an Arrhenius acid and an Arrhenius base is called neutralisation and results in the formation of water and a salt.
- A Brønsted-Lowry acid is a proton donor; a Brønsted-Lowry base is a proton acceptor.
- Acid-base reactions include two sets of conjugate acid-base pairs.
Exercises

