1 8.0 What is Organic Chemistry?
What is Organic Chemistry?
Organic chemistry is a branch of chemistry that focuses on the study of carbon-containing compounds. While it initially dealt primarily with compounds derived from living organisms (hence the term “organic”), the definition has evolved to include a vast array of synthetic compounds as well. Carbon is a unique element in that it can form stable and diverse structures through covalent bonding with other carbon atoms and various elements.
Nature is filled with chemical structures of many types, but in the chemistry of life, we find an abundance of organic chemicals. Carbon has an outsized role to play in life and, thus, in our chemical activities. Agriculture, manufactured goods from ag products (like textiles), and pharmaceutical products are all largely based on organic molecules. The petroleum industry and all the products related to that are also linked to organic chemistry because petroleum is a fossil fuel produced in geologic processes from formerly living matter. In modern times, these substances are the raw materials converted into a huge variety of plastics that we use in constructing the built world.
Why Carbon?
Carbon, atomic number 6, is an element with atoms that are small and relatively simple. Its nucleus contains 6 positively charged protons, and there are 6 electrons outside the nucleus distributed into two shells. The outer shell has four electrons that are held quite strongly by the electrostatic pull from the nucleus. So, while a carbon atom can be ionised through either the gain or loss of electrons, it does not tend to do so. Carbon does, however, readily engage in covalent bonding, sharing electrons with neighbouring atoms and forming tight associations with them. The four valence electrons in a carbon atom can do this by forming four single bonds, by forming two single bonds and a double bond, by forming one single bond and a triple bond, or by forming two double bonds.
Carbons also covalently bond with one another, forming chains of various lengths and rings. It readily bonds with other atoms, such as oxygen, nitrogen and hydrogen, forming quite stable arrangements with these common elements as well.
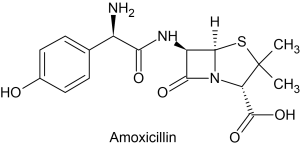
The architecture of carbon structures is, therefore, fantastically varied. Small organic molecules might contain just one or two carbon atoms surrounded by other atoms. But the larger organic molecules can contain hundreds or thousands of carbons, linked with rings and bridges and other complex structures that fold into particular three-dimensional structures. Figure 8.0.2 shows the chemical structure of the antibiotic amoxicillin, which consists of several carbon atoms forming single and double bonds and rings.
No other element can quite do what carbon does: silicon has the ability to form four bonds with other atoms, but those bonds tend to be weak due to the additional electron shell in a silicon atom. Nitrogen has five valence electrons, so it generally only forms 3 single bonds, limiting its usefulness. Boron, similarly, does not make for a dependable, stable base structure.

