2.1 Atoms: The Building Blocks of Everything
Learning Objectives
- State the modern atomic theory.
- Learn how atoms are constructed.
- Define the terms atoms, molecules and compounds.
Atoms and Elements
Within the chemical world, atoms are the essential building blocks, composing the many molecules and compounds we interact with. All atoms consist of a small, positively charged nucleus that is made up of positively charged protons, as well as neutrons, which themselves have no charge. This is surrounded by a larger, negatively charged electron cloud (Figure 2.1.1). They are too small to be observed by the naked eye.
The concept that atoms play a fundamental role in chemistry is formalised by modern atomic theory, first stated by John Dalton, an English scientist, in 1808. It consists of three parts:
- All matter is composed of atoms.
- Atoms of the same element are the same; atoms of different elements are different.
- Atoms combine in whole-number ratios to form compounds.
These concepts form the basis of chemistry.
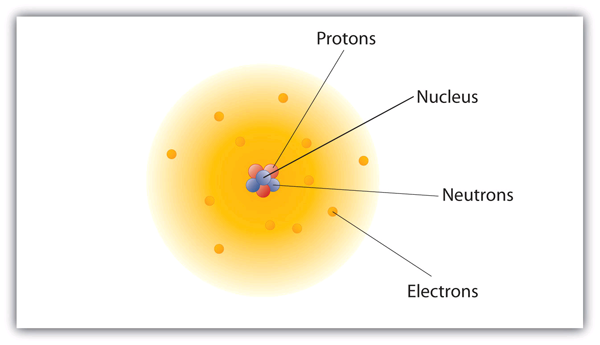
Although the word atom comes from a Greek word that means “indivisible,” we now understand that atoms themselves are composed of smaller parts called subatomic particles. The subatomic particles present in an atom’s nucleus—protons and neutrons—are collectively known as nucleons.. Protons and neutrons are almost identical in mass, whereas electrons are much lighter. Protons hold a positive (+1) charge, while electrons hold a negative (-1) charge. The properties of the subatomic particles are given in Table 2.1.1. Atoms are generally neutral species due to the presence of an equal number of protons and electrons.
| Subatomic particle | Symbol | Charge | Mass (g) | Size relative to a proton |
|---|---|---|---|---|
| Proton | p | [latex]+1[/latex] | [latex]1.673\times10^{-24}[/latex] | [latex]1[/latex] |
| Neutron | n | [latex]0[/latex] | [latex]1.675\times10^{-24}[/latex] | [latex]1[/latex] |
| Electron | e | [latex]-1[/latex] | [latex]9.109\times10^{-28}[/latex] | [latex]\frac{1}{1800}[/latex] |
Atoms come in many different “elements” — depending on the number of protons present. Each element has a unique name and a chemical symbol. We will learn how to read the periodic table in 2.2 ‘Electronic Configuration’, to determine the name of a given element. Examples of element symbols are given in Table 2.1.2.
| Element | Chemical symbol |
|---|---|
| Hydrogen | [latex]\ce{H}[/latex] |
| Carbon | [latex]\ce{C}[/latex] |
| Calcium | [latex]\ce{Ca}[/latex] |
| Silver | [latex]\ce{Ag}[/latex] |
| Copper | [latex]\ce{Cu}[/latex] |
Elements act as the foundations of all matter. Some elements naturally exist as multiatomic, for instance, [latex]\ce{S8}[/latex], [latex]\ce{O2}[/latex], and [latex]\ce{Cl2}[/latex] while others such as [latex]\ce{He}[/latex], [latex]\ce{Ar}[/latex], and, [latex]\ce{Ne}[/latex] naturally stand alone. This can be predicted by observing the electrons present, which will be explained in greater detail in the next chapter.
As atoms can have differing numbers of subatomic particles we utilise atomic and mass numbers to determine their amount. The atomic number (Z) is the number of protons present in the nucleus of an atom.
Atomic number (Z) = Number of protons
The mass number (A) of an atom is the sum of the number of protons and neutrons present in the nucleus of an atom.
Mass number (A) = Number of protons + Number of neutrons
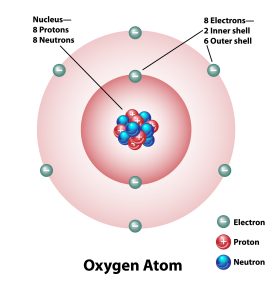
Let’s observe how these formulae can be used to determine the atomic number and mass number of a standard oxygen atom, as illustrated in Figure 2.1.2. Within the oxygen atom, we have 8 protons, 8 neutrons and 8 electrons.
Atomic number (Z) = Number of protons = 8
Mass number (A) = Number of protons + Number of neutrons = 8 + 8 = 16.
It can, therefore, be seen that a standard oxygen atom has an atomic number of 8 and a mass number of 16. We represent this as [latex]\ce{_{8}^{16}O}[/latex].
The relationship between atomic numbers, mass numbers and the number of protons and neutrons present within an atom allows for a variety of problem-solving equations. For instance, one can work out the atomic number of an atom from the number of neutrons and the mass number alone.
Molecules and Compounds
While this chapter focuses on atoms by themselves, important terminology should be established. The terms atoms, molecules and compounds can’t be used interchangeably. A molecule is a group of two or more atoms combined chemically and functions as a unit. The simplest molecule that can exist is a diatomic molecule that contains two atoms, such as [latex]\ce{O2}[/latex] and [latex]\ce{H2}[/latex]. Compounds contain different types of atoms in fixed proportions. Compounds and molecules are uncharged, neutral species.
Chemical formulas consist of chemical symbols of the elements present in the compound and numerical subscripts, which demonstrate the number of atoms of each element involved in the formation of the compound.
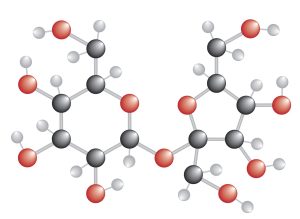
- The chemical formula for the compound sucrose is [latex]\ce{C12H22O11}[/latex]
This formula shows that sucrose contains three different elements: [latex]\ce{C\;H\;O}[/latex]
Total number of atoms: 45
Number of each type of atom: [latex]\ce{C} - 12, \ce{H} - 22, \ce{O} - 11[/latex] - The chemical formula for the compound calcium phosphate is [latex]\ce{Ca3(PO4)2}[/latex]
This formula shows that one unit of calcium phosphate is composed of three different elements: [latex]\ce{Ca\;P\;O}[/latex]
Total number of atoms: 13
Number of each type of atom: [latex]\ce{Ca} - 3, \ce{P} - 2, \ce{O} - 8[/latex] (Note: as there are two phosphate ions ([latex]\ce{PO4}[/latex]), for [latex]\ce{O}[/latex]and [latex]\ce{P}[/latex] multiply the subscript number by the number written after the parenthesis.)
Key Takeaways
- Atoms are small particles comprised of positively charged protons, neutral neutrons, and negatively charged electrons.
- Atoms come in many different elements, denoted by symbols found on the periodic table (such as [latex]\ce{H}[/latex] or [latex]\ce{Cu}[/latex]).
- The atomic number of a particle reflects the number of protons it contains, while the mass number is the full amount of protons and neutrons added together.
- Chemical formulae tell you how many of which elements are involved in the makeup of a compound
Exercises
Practice Questions
Media Attributions
- Diagram of an oxygen atom with nucleus and inner and outer shells. Protons, neutrons, and electrons are labeled. © O Sweet Nature - stock.adobe.com is licensed under a All Rights Reserved license
- Molecular formula of sucrose. Sucrose or tea sugar is a disaccharide formed by the combination of a glucose and a fructose molecule. © Firat - stock.adobe.com is licensed under a All Rights Reserved license
The smallest piece of an element that maintains the identity of that element.
A unit of two or more same elements bonded together.
A subatomic particle with a positive charge.
A subatomic particle with no charge.
A tiny subatomic particle with a negative charge.
Particles which make up atoms: protons, neutrons, and electrons.
A system where the positive and negative charges cancel each other out
The one/two letter shorthand version of referring to a specific element. Used in chemical formulas.
A substance that cannot be broken down into simpler chemical substances by ordinary chemical means.
The number of protons in an atom.
The sum of the number of protons and neutrons in a nucleus.
A molecule consisting of only two (di-) atoms.
A combination of more than one element.
The chemical composition of a molecule or compound.

