7.5 Rates of Reaction
Learning Objectives
- Learn about the requirements for a reaction to occur under collision theory: sufficient energy and correct orientation.
- Learn about how reactant concentration, surface area, temperature and the presence of a catalyst can alter the rate of a chemical reaction.
Introduction
Speed plays an important role in many things we do in everyday life. If you sleep in and need to get to your chemistry lecture quickly, you may choose to drive instead of walk because driving is faster. You may use a favourite website to stream video content online because of its quick and reliable download speeds. When applying for a summer job, you may have had to include your typing speed on your resume to show your competency using computers. These examples emphasise that the speed of a process is an important consideration in our everyday lives. Similarly, the speed of a chemical reaction is also a significant consideration and is called its reaction rate. Reaction rates vary dramatically, with some reactions occurring on a time scale of seconds, while other reactions take many thousands of years. Several factors can influence reaction rate, and the study of the interplay between these factors and the rate of a chemical reaction is called kinetics.
Factors that Affect the Rate of Reactions
Reaction kinetics is the study of the rate of chemical reactions, and reaction rates can vary greatly over a large range of time scales. Some reactions can proceed at explosively fast rates, like the detonation of fireworks (see Figure 7.5.1), while others can occur at a sluggish rate over many years, like the rusting of barbed wire exposed to the elements (see Figure 7.5.2).


Collision Theory Expanded
Let us expand our understanding of collision theory further. While we have already discussed how temperature increases the kinetic speed of molecules, we must also consider how chemical reactions begin. It is not only enough for molecules to touch — chemical processes only occur when reactant molecules “effectively collide.” For an “effective collision” to occur, the reactant molecules must be oriented in space correctly to facilitate the breaking and forming of bonds and the rearrangement of atoms that result in the formation of product molecules (see Figure 7.5.3).
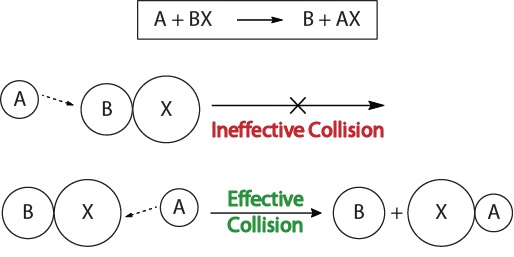
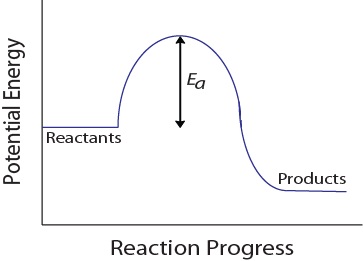
During a molecular collision, molecules must also possess a minimum amount of kinetic energy for an effective collision to occur. This requirement varies for each reaction and is known as the activation energy (Ea) (see Figure 7.5.4). The rate of reaction, therefore, depends on the activation energy; a higher activation energy means that fewer molecules will have sufficient energy to undergo an effective collision.
Factors That Affect Rate
There are four main factors that can affect the reaction rate of a chemical reaction:
- Reactant concentration. Increasing the concentration of one or more reactants will often increase the rate of reaction. This occurs because a higher concentration of a reactant increases the number of molecules within the same space and will lead to more collisions of that reactant in a specific time period.
- Physical state of the reactants and surface area. If reactant molecules exist in different phases, as in a heterogeneous mixture, the reaction rate will be limited by the surface area of the phases in contact. For example, if a solid metal reactant and gas reactant are mixed, only the molecules present on the surface of the metal are able to collide with the gas molecules. Therefore, increasing the surface area of the metal by pounding it flat or cutting it into many pieces will increase its reaction rate. This is why sugar cubes dissolve slower than powdered sugar.
- Temperature. An increase in temperature typically increases the rate of reaction. An increase in temperature will raise the average kinetic energy of the reactant molecules. Therefore, a greater proportion of molecules will have the minimum energy necessary for an effective collision (see Figure 7.5.5).
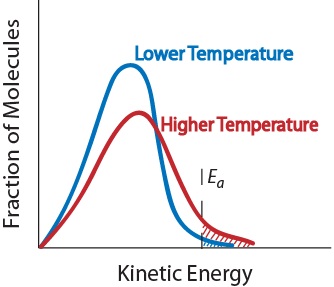
Figure 7.5.5 “Temperature and Reaction Rate.” Effect of temperature on the kinetic energy distribution of molecules in a sample. Transcript. Image attribution: Chem&121: Introduction to Chemistry Copyright © 2023 by Lake Washington Institute of Technology is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. - Presence of a catalyst. A catalyst is a substance that accelerates a reaction by participating in it without being consumed. Catalysts provide an alternate reaction pathway to obtain products — that has a lower activation energy than the catalyst-free pathway. They are critical to many biochemical reactions.
Key Takeaways
- Reaction kinetics is the study of the rate of chemical reactions.
- According to collision theory, for a reaction to occur, molecules must collide with one another with sufficient energy and at the correct orientation.
- Collision theory allows us to predict how a variety of factors speed up chemical reactions, such as reactant concentration, surface area, temperature, and catalysts.
Exercises
A compound that lowers activation energy and increases rate of reaction that isn't consumed.

