2.3 The Periodic Table
Learning Objectives
- Relate the electron configurations of the elements to the shape of the periodic table.
- Determine the expected electron configuration of an element by its place on the periodic table.
To organise the various elements in chemistry, the periodic table arranges elements in order of increasing atomic number. The rows and columns in the periodic table are known as periods and groups, respectively (see Figure 2.3.1).
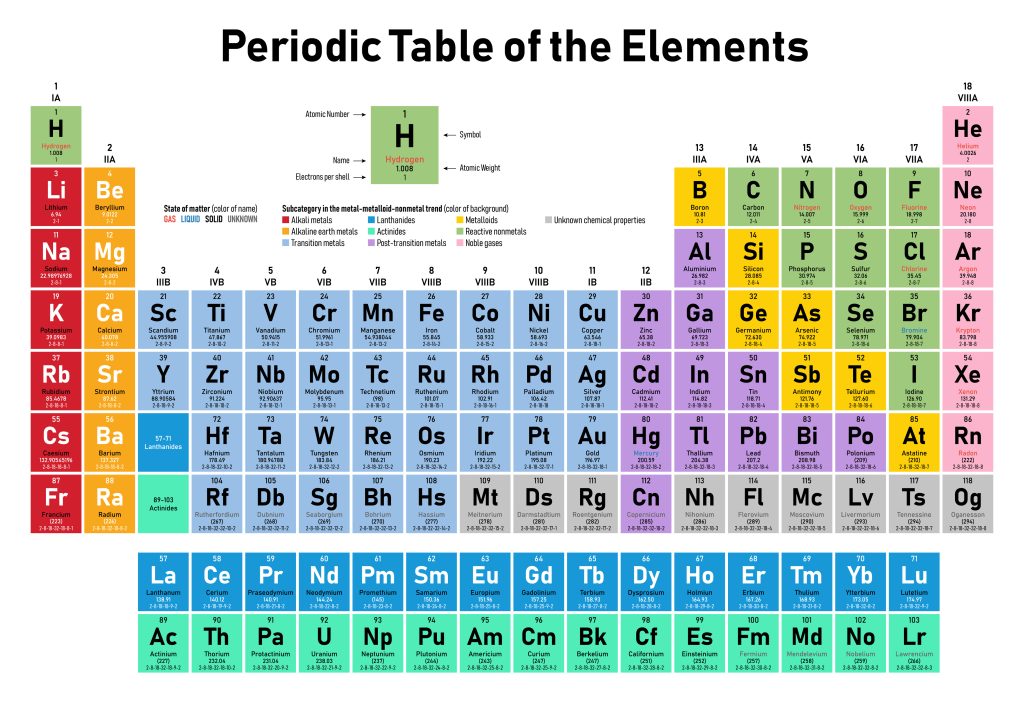
Why does the periodic table have the structure it does? The answer is rather simple if you understand electron configurations: the shape of the periodic table mimics the filling of the subshells with electrons.
Dividing the table by electronic subshells
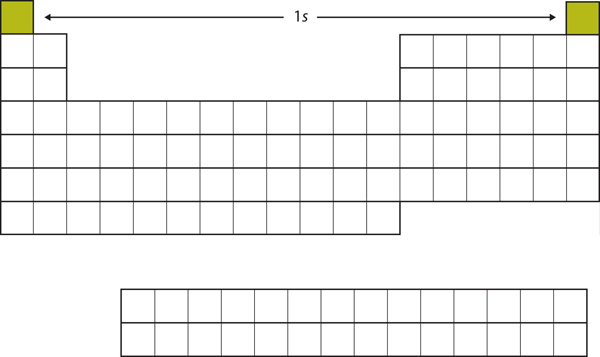
Let us start with [latex]\ce{H}[/latex] and [latex]\ce{He}[/latex]. Their electron configurations are [latex]1s^1[/latex]and [latex]1s^2[/latex], respectively; with [latex]\ce{He}[/latex], the n = 1 shell is filled. These two elements make up the first row of the periodic table (see Figure 2.3.2 “The 1s Subshell”).
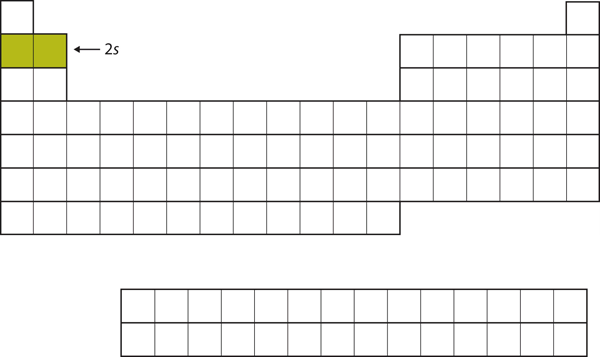
The next two electrons, for [latex]\ce{Li}[/latex] and [latex]\ce{Be}[/latex], would go into the [latex]2s[/latex] subshell. Figure 2.3.3 “The 2s Subshell” shows that these two elements are adjacent on the periodic table.
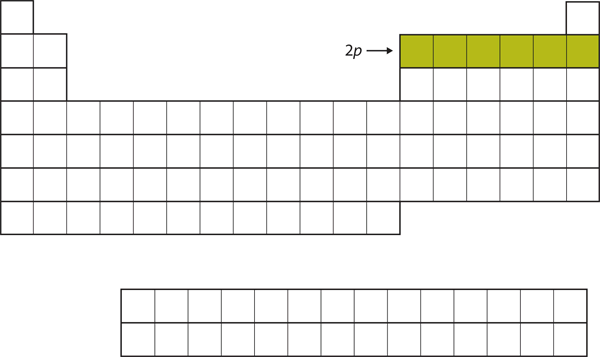
For the next six elements, the [latex]2p[/latex] subshell is being occupied with electrons. On the right side of the periodic table, these six elements ([latex]\ce{B}[/latex] through [latex]\ce{Ne}[/latex]) are grouped together (Figure 2.3.4 “The 2p Subshell”).
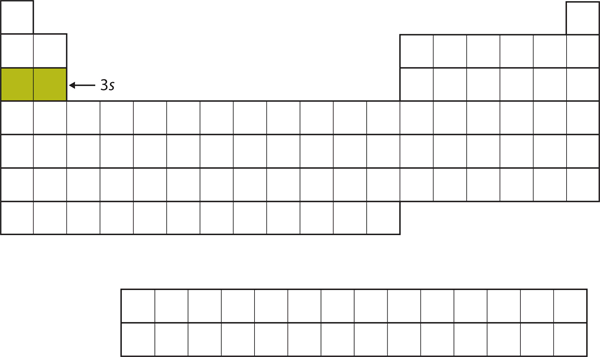
The [latex]3s[/latex] subshell is then filled. The elements when this subshell is being filled, [latex]\ce{Na}[/latex] and [latex]\ce{Mg}[/latex], are on the left side of the periodic table (Figure 2.3.5 “The 3s Subshell”).
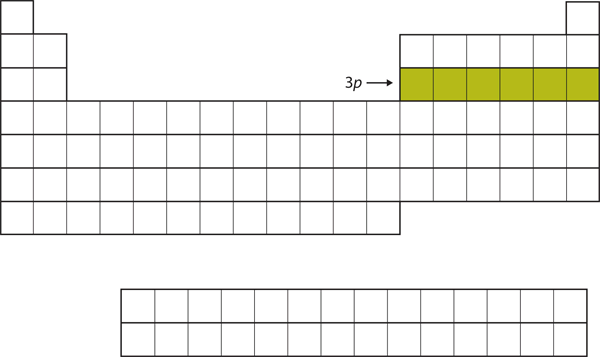
Next, the [latex]3p[/latex] subshell is filled with the next six elements (Figure 2.3.6 “The 3p Subshell”).
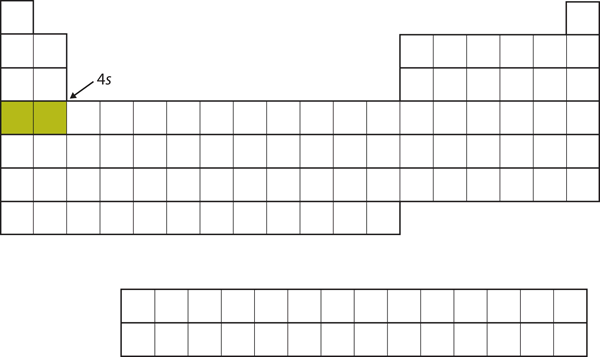
Recall 2.2 ‘Electronic Configuration’ and the electron subshell filling orders. Instead of filling the [latex]3d[/latex] subshell next, electrons go into the [latex]4s[/latex] subshell, which consists of [latex]\ce{K}[/latex] and [latex]\ce{Ca}[/latex] (Figure 2.3.7 “The 4s Subshell”).
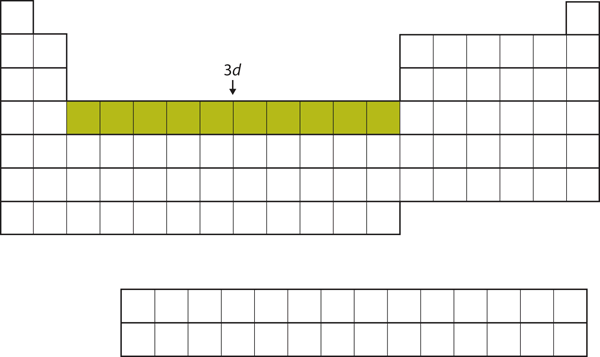
After the [latex]4s[/latex] subshell is filled, the [latex]3d[/latex] subshell is filled with up to 10 electrons. This explains the section of 10 elements in the middle of the periodic table, which consists of [latex]\ce{Sc}[/latex] to [latex]\ce{Zn}[/latex] (Figure 2.3.8 “The 3d Subshell”).
And so forth. As this process continues, we go across the rows of the periodic table, with the overall shape of the table outlining how the electrons occupy the shells and subshells.
Dividing the table by shells
The first two columns on the left side of the periodic table are where the [latex]s[/latex] subshells are occupied. Because of this, the first two columns of the periodic table are labelled the [latex]s[/latex] block. Similarly, the [latex]p[/latex] block is located in the right-most six columns of the periodic table, the [latex]d[/latex] block is the middle 10 columns of the periodic table, while the [latex]f[/latex] block is the 14-column section that is normally depicted as detached from the main body of the periodic table. It could be part of the main body, but then the periodic table would be rather long and cumbersome. Figure 2.3.9 “Blocks on the Periodic Table” shows the blocks of the periodic table.
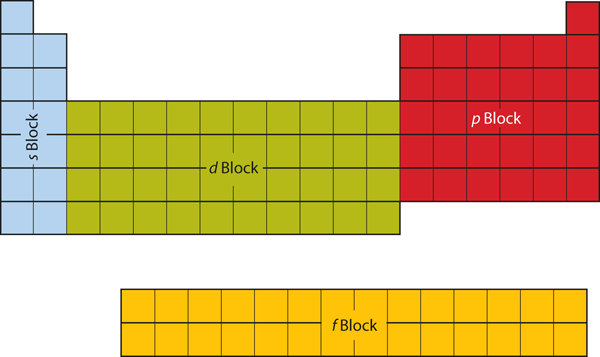
The electrons in the highest-numbered shell, plus any electrons in the last unfilled subshell, are called valence electrons; the highest-numbered shell is called the valence shell. (The inner electrons are called core electrons.) The valence electrons largely control the expected chemistry and reactive properties of an atom. To illustrate, we find that in each column of the periodic table, the valence shell’s electron configuration is the same. Take the elements in the first column of the periodic table: [latex]\ce{H}[/latex], [latex]\ce{Li}[/latex], [latex]\ce{Na}[/latex], [latex]\ce{K}[/latex], [latex]\ce{Rb}[/latex], and [latex]\ce{Cs}[/latex]. Their electron configurations (abbreviated for the larger atoms) are as follows, with the valence shell electron configuration highlighted:
| Element | Electron Configuration |
|---|---|
| H | 1s1 |
| Li | 1s22s1 |
| Na | [Ne]3s1 |
| K | [Ar]4s1 |
| Rb | [Kr]5s1 |
| Cs | [Xe]6s1 |
They all have a similar electron configuration in their valence shells: a single [latex]s[/latex] electron. Because much of the chemistry of an element is influenced by valence electrons, we would expect that these elements would have similar chemistry — and they do. The organisation of electrons in atoms explains not only the shape of the periodic table but also the fact that elements in the same column of the periodic table have similar chemistry.
Dividing the table by chemical properties
The same concept applies to the other columns of the periodic table. Elements in each column have the same valence shell electron configurations, and the elements have some similar chemical properties. This is strictly true for all elements in the [latex]s[/latex] and [latex]p[/latex] blocks. In the [latex]d[/latex] and [latex]f[/latex] blocks, because there are exceptions to the order of filling of subshells with electrons, similar valence shells are not absolute in these blocks. However, many similarities do exist in these blocks, so a similarity in chemical properties is expected. To this extent, the groups can be further classified as seen in Table 2.3.2.
| Group | Name | Examples | Chemical properties |
|---|---|---|---|
| 1 ([latex]IA[/latex]) | Alkali metals | [latex]\ce{Li,\,Na,\,K}[/latex] | Soft and shiny metals, highly reactive with water |
| 2 ([latex]IIA[/latex]) | Alkaline earth metals | [latex]\ce{Be,\,Mg,\,Ca}[/latex] | Soft and shiny metals, moderately reactive with water |
| 17 ([latex]VIIA[/latex]) | Halogens | [latex]\ce{F,\,Cl,\,Br}[/latex] | Generally, reactive elements, exist as gases at room temperature |
| 18 ([latex]VIIIA[/latex]) | Noble gases | [latex]\ce{He,\,Ne,\,Ar}[/latex] | Exist as gases, unreactive elements |
Based on selected physical properties, elements are further classified into metals and nonmetals. Metals are located on the left side of the periodic table, and nonmetals are located on the right. Metals are good conductors of electricity and heat; exist as solids at room temperature (except mercury); are ductile; malleable; have shiny appearances (metallic lustre); and have high density and high melting points.
Elements known as metalloids exhibit both metallic and nonmetallic properties. Metalloids are located between metals and nonmetals in the periodic table.
Key Takeaways
- The arrangement of electrons in atoms is responsible for the shape of the periodic table.
- Electron configurations can be predicted by the position of an atom on the periodic table.
- Elements within the same group or column have similar valence electron shell configurations – often exhibiting similar properties as a result.
Exercises
Media Attributions
Electrons found on the outer shell of an atom.
The highest-numbered shell in an atom that contains electrons.
Able to be drawn into a thin wire.
Easy to mold; won't shatter under pressure and force.

