3.4 Metallic Bonds
Learning Objectives
- Define the properties of metallic bonds and delocalised electrons
- Understand the general characteristics of metals.
To complete our types of bonding, metallic bonds present when metals are bonded to other metals together in a lattice. Metallic bonds exhibit unique sets of properties within compounds, all due to a sea of delocalised electrons (see Figure 3.4.1).
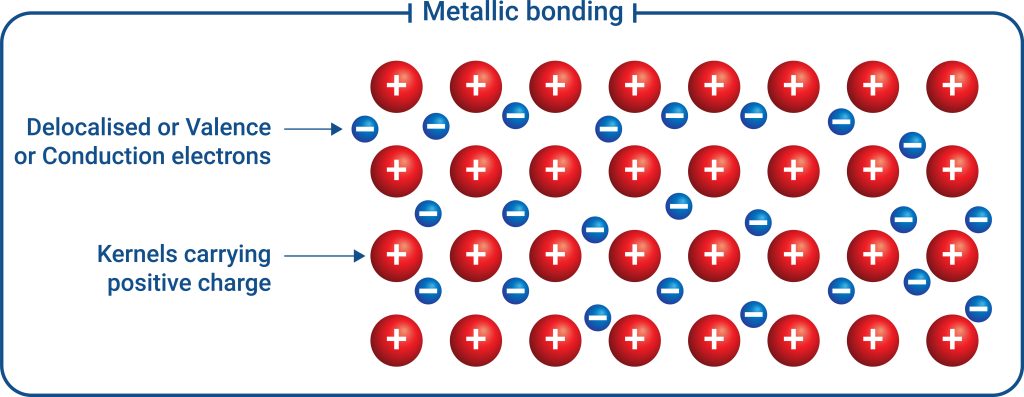

In covalent and ionic bonds, the electron is tied between one or two atoms. Within metallic bonds, valence electrons are essentially liberated from any individual atom due to electron orbitals overlapping, allowing electrons to flow freely within the lattice. These delocalised electrons require significantly less energy to shift around due to a lack of attraction forces to the nucleus. Even with just a small current, electrons will readily move throughout the structure. This makes metals fantastic conductors of electricity and heat!
The metallic lattice that forms and delocalised electrons cause metals to be malleable and ductile. Metals that are subjected to forces are able to shift their atoms whilst retaining the delocalised electrons and the lattice structure.
Metals are generally characterised as having high melting points and being lustrous, paramagnetic, and solid at room temperature; however, the individual electronic configuration of metallic elements does cause exceptions to arise. Mercury, for instance, is a transition metal but is a liquid at room temperature.
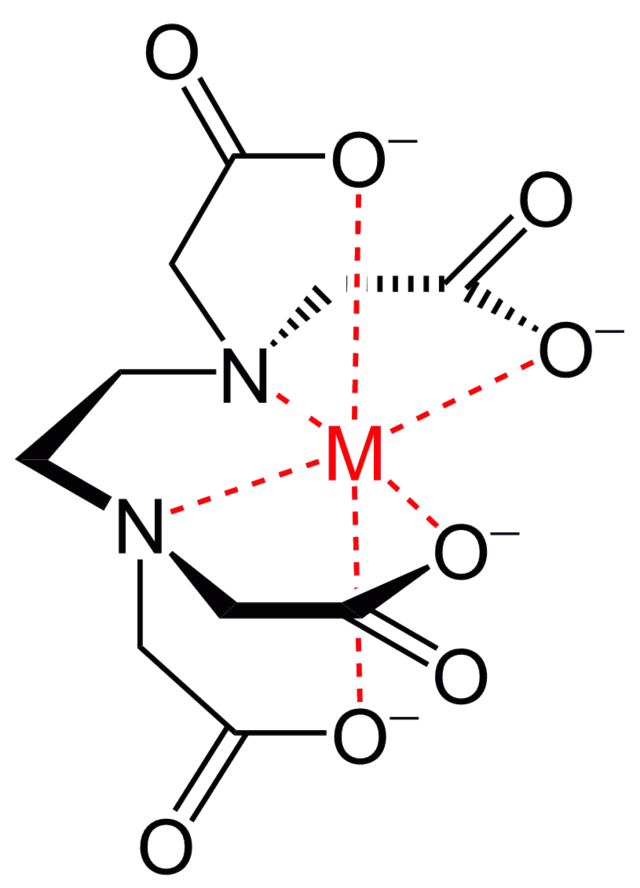
You may notice we are glossing over metallic bonding! We stress that students are aware of the general characteristics of metals, being delocalised electrons leading to the high conduction of electricity and heat alongside high melting points due to the rigid lattice. You’ll often be using hotplates or other metal implements in the lab, so understanding why they work is important! However, tertiary chemistry studies don’t focus on traditional metallic bonding. The study of inorganic chemistry focuses on the formation of metal complexes through bonding with ‘ligands’ that attract metal ions. Inorganic chemistry requires its own separate field of study, as metals can form many more bonds than would be traditionally expected (see Figure 3.4.3).
Inorganic chemistry often deals with how the oxidation states and coordination of transition metals change with respect to pH, heat and other variables. These oxidation states often produce energy changes with wavelengths within the visible spectrum, making inorganic chemistry one of the more colourful and exciting fields (see Figure 3.4.4)! For now, however, this study is outside the scope of this textbook.

Key Takeaways
- Metallic bonding occurs when metals are bonded in a lattice.
- Metallic bonds feature a sea of delocalised electrons due to overlapping electron orbitals.
- Delocalised electrons allow for the easy conduction of heat and electricity compared to other elements and compounds.
Exercises
Media Attributions
An element that conducts electricity and heat well and is shiny, silvery, solid, ductile, and malleable.
3D crystalline structure of a compound which forms and can be continuously built upon.
The specific set of principal, angular momentum, and magnetic quantum numbers for an electron.
Easy to mold; won't shatter under pressure and force.
Able to be drawn into a thin wire.

