7.3 Exothermic and Endothermic Reactions
Learning Objectives
- Define what constitutes an exothermic and endothermic chemical reaction.
- Learn about enthalpy and the expected change in exothermic and endothermic reactions.
All reactions involve the rearrangement of chemical bonds within molecules. The bonds that form as a result of a chemical reaction will hold either more or less chemical energy than the initial reactants. This is expressed as heat. You will most likely be familiar with exothermic and endothermic reactions throughout your day-to-day life — distinguished through how energy is released or absorbed.
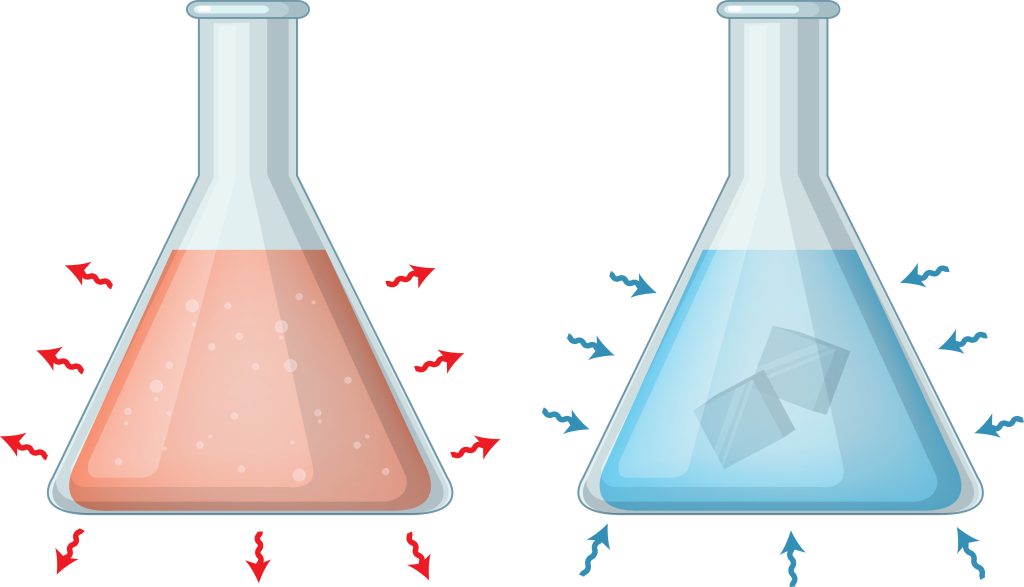
Exothermic reactions are those that release energy into the environment. Remember how the law of conservation of energy requires that energy be maintained? In an exothermic reaction, the chemical energy of products is less than that of the starting reactants. This difference in energy is expressed as heat. This is the theory behind instant heat packs. When activating, reactants can interact and transform into end products with lower chemical energies. Heat is produced as a result, allowing the soothing of injuries and aches on one’s body.
In contrast, endothermic reactions absorb energy from the environment. When the reactants come into contact, the final product has chemical energy demands greater than the reactants — meaning it must pull in energy from its surroundings. This results in a cold sensation when touched.
Enthalpy
An important concept to chemists is the amount of energy gained or lost through chemical reactions (such as combustion). It is vital, therefore, that we define a new concept to measure this phenomenon: enthalpy.
Enthalpy ([latex]\ce{H}[/latex]) is the amount of energy found within a system (at constant pressure). Within the context of thermochemistry, we are concerned with how chemical energy transforms into thermal energy. Knowing that exothermic and endothermic reactions depend on the change of chemical energy between reactants and products, we can determine if a reaction is endothermic or exothermic in nature by observing the change in enthalpy. Let us take the combustion of propane for example (cited from W.M.Haynes CRC Handbook of Chemistry 97th Edu.):
[latex]\ce{C3H8 + 5O2 -> 3CO2 + 4H2O~~~\Delta H = -2220 kJ/mol}[/latex]
The combustion of propane produces a negative enthalpy, denoted by [latex]\ce{\Delta H}[/latex]. This means that compared to the reactants, the final products possess less energy. This energy is released in the form of heat. As such, a negative [latex]\ce{\Delta H}[/latex] informs us that the reaction is exothermic. Conversely, a positive [latex]\ce{\Delta H}[/latex] indicates an endothermic reaction. For this equation, every mole of propane combusted releases 2220 kilojoules of energy to the environment.
Key Takeaways
- Chemical reactions are either endothermic (draw energy) or exothermic (release energy).
- A chemical reaction is exothermic if the chemical energy of products is lower than that of the starting reactants, while an endothermic reaction occurs when the products’ energy is higher.
- Enthalpy is the measure of energy found within a molecule or system. The change in enthalpy allows us to determine the amount of energy drawn or released from a reaction.
Exercises
Media Attributions
- Types of chemical reactions Exothermic and endothermic reactions © Nandalal - stock.adobe.com is licensed under a All Rights Reserved license
A chemical reaction that has a negative change in enthalpy.
A chemical reaction that has a positive change in enthalpy.
Law of physics that states that the total energy of an isolated system does not increase or decrease.
The energy found within chemical bonds in a compound.
End result of a chemical reaction.
Beginning substances of a chemical reaction.
Amount of energy within a system under constant pressure.

