4.5 Ionic Equations
Learning Objectives
- Write ionic equations for chemical reactions between ionic compounds.
- Recognise spectator ions.
- Write net ionic equations for chemical reactions between ionic compounds.
As we learned in Chapter 3, ionic compounds are compounds between metals and nonmetals or compounds that contain recognisable polyatomic ions. Now, we take a closer look at reactions that include ionic compounds.
An important distinction between ionic compounds and molecular compounds is how they dissolve in a liquid such as water. When molecular compounds, such as sugar, dissolve in water, the individual molecules drift apart from each other. When ionic compounds dissolve, the ions physically separate from each other. We can use a chemical equation to represent this process—for example, with [latex]\ce{NaCl}[/latex]:
[latex]\ce{NaCl(s)}\xrightarrow{\ce{H2O}}\ce{Na^+(aq)}+\ce{Cl^-(aq)}[/latex]
When [latex]\ce{NaCl}[/latex] dissolves in water, the ions separate and go their own way in the solution; the ions are now written with their respective charges, and the (aq) phase label emphasises that they have been dissolved (Figure 4.3.1 “Ionic Solutions”). This process is called dissociation; we say that the ions dissociate.
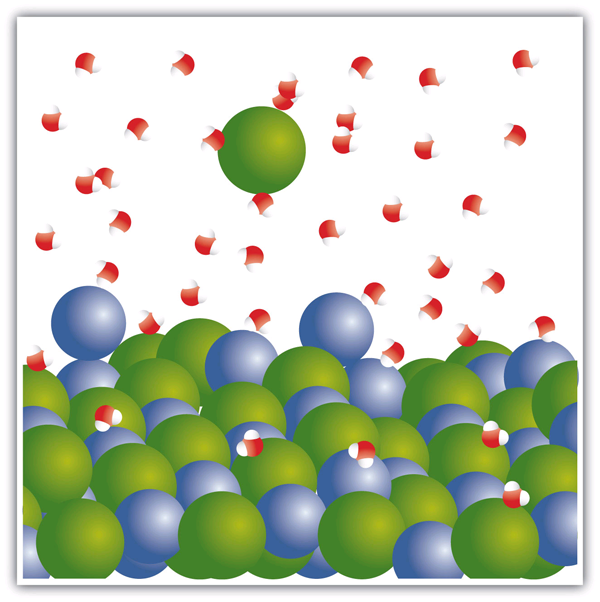
The behaviour of ionic compounds was first suggested by the Swedish chemist Svante August Arrhenius (1859–1927) as part of his PhD dissertation in 1884. Interestingly, his PhD examination team had a hard time believing that ionic compounds would behave like this, so they gave Arrhenius a barely passing grade. Later, this work was cited when Arrhenius was awarded the Nobel Prize in Chemistry.
All ionic compounds that dissolve behave this way. Keep in mind that when the ions separate, all the ions separate. Thus, when [latex]\ce{CaCl}_{2}[/latex] dissolves, the one [latex]\ce{Ca}^{2+}[/latex] ion and the two [latex]\ce{Cl}^{-}[/latex] ions separate from each other:
[latex]\begin{gather*} \ce{CaCl2(s)}\xrightarrow{\ce{H2O}}\ce{Ca^{2+}(aq)}+\ce{Cl^-(aq)}+\ce{Cl^-(aq)} \\ \text{or} \\ \ce{CaCl2(s)}\xrightarrow{\ce{H2O}}\ce{Ca^{2+}(aq)}+\ce{2Cl^-(aq)} \end{gather*}[/latex]
That is, the two chloride ions go off on their own. They do not remain as [latex]\ce{Cl}_{2}[/latex] (that would be elemental chlorine; these are chloride ions); they do not stick together to make [latex]\ce{Cl}_{2}^{-}[/latex] or [latex]\ce{Cl}_{2}^{2-}[/latex]. They become dissociated ions in their own right. Polyatomic ions also retain their overall identity when they are dissolved.
Example 4.5.1
Problems
Write the chemical equation that represents the dissociation of each ionic compound.
- [latex]\ce{KBr}[/latex]
- [latex]\ce{Na}_{2}\ce{SO}_{4}[/latex]
Solution
- [latex]\ce{KBr}{(s)} {\rightarrow} \ce{K}^{+}{(aq)} + \ce{Br}^{-}{(aq)}[/latex]
- Sodium ions go their own way, but the sulphate ion stays together as the sulphate ion. The dissolving equation is:
[latex]\ce{Na}_{2}\ce{SO}_{4}{(s)} → \ce{2Na}^{+}{(aq)} + \ce{SO}_{4}^{2-}{(aq)}[/latex]
Test Yourself
Write the chemical equation that represents the dissociation of [latex]\ce{(NH_{4})_{2}S}[/latex].
Answer
[latex]\ce{(NH_{4})_{2}S}{(s)} {\rightarrow} \ce{2NH}_{4}^{+}{(aq)} + \ce{S}^{2-}{(aq)}[/latex]
When chemicals in solution react, the proper way of writing the chemical formulas of the dissolved ionic compounds is in terms of the dissociated ions, not the complete ionic formula. A complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. For example, when [latex]\ce{NaCl}{(aq)}[/latex] reacts with [latex]\ce{AgNO}_{3}{(aq)}[/latex] in a double-replacement reaction to precipitate [latex]\ce{AgCl}{(s)}[/latex] and form [latex]\ce{NaNO}_{3}{(aq)}[/latex], the complete ionic equation includes [latex]\ce{NaCl}[/latex], [latex]\ce{AgNO}_{3}[/latex], and [latex]\ce{NaNO}_{3}[/latex] written as separated ions:
[latex]\ce{Na}^{+}{(aq)} + \ce{Cl}^{-}{(aq)} + \ce{Ag}^{+}{(aq)} + \ce{NO}_{3}^{-}{(aq)} {\rightarrow} \ce{AgCl}{(s)} + \ce{Na}^{+}{(aq)} + \ce{NO}_{3}^{-}{(aq)}[/latex]
This is more representative of what is occurring in the solution.
Example 4.5.2
Problems
Write the complete ionic equation for each chemical reaction.
- [latex]\ce{KBr}{(aq)} + \ce{AgC}_{2}\ce{H}_{3}\ce{O}_{2}{(aq)} {\rightarrow} \ce{KC}_{2}\ce{H}_\ce{3}{O}_{2}{(aq)} + \ce{AgBr}{(s)}[/latex]
- [latex]\ce{MgSO}_{4}{(aq)} + \ce{Ba(NO_{3})}_{2}{(aq)} {\rightarrow} \ce{Mg(NO_{3})}_{2}{(aq)} + \ce{BaSO}_{4}{(s)}[/latex]
Solution
For any ionic compound that is aqueous, we write the compound as separated ions.
- The complete ionic equation is:
[latex]\ce{K}^{+}{(aq)} + \ce{Br}^{-}{(aq)} + \ce{Ag}_{+}{(aq)} + \ce{C}_{2}\ce{H}_{3}\ce{O}_{2}^{-}{(aq)} {\rightarrow} \ce{K}^{+}{(aq)} + \ce{C}_{2}\ce{H}\ce{O}_{2}^{-}{(aq)} + \ce{AgBr}{(s)}[/latex]
- The complete ionic equation is:
[latex]\ce{Mg}^{2+}{(aq)} + \ce{SO}_{4}^{2-}{(aq)} + \ce{Ba}^{2+}{(aq)} + \ce{2NO}_{3}^{-}{(aq)} {\rightarrow} \ce{Mg}^{2+}{(aq)} + \ce{2NO}_{3}^{-}{(aq)} + \ce{BaSO}_{4}{(s)}[/latex]
Test Yourself
Write the complete ionic equation for:
[latex]\ce{CaCl}_{2}{(aq)} + \ce{Pb(NO_{3})}_{2}{(aq)} {\rightarrow} \ce{Ca(NO_{3})}_{2}{(aq)} + \ce{PbCl}_{2}{(s)}[/latex]
Answer
[latex]\ce{Ca}_{2+}{(aq)} + \ce{2Cl}^{-}{(aq)} + \ce{Pb}^{2+}{(aq)} + \ce{2NO}_{3}^{-}{(aq)} {\rightarrow} \ce{Ca}^{2+}{(aq)} + \ce{2NO}_{3}^{-}{(aq)} + \ce{PbCl}_{2}{(s)}[/latex]
You may notice that in a complete ionic equation, some ions do not change their chemical form; they stay exactly the same on the reactant and product sides of the equation. For example, in:
[latex]\ce{Na}^{+}{(aq)} + \ce{Cl}^{-}{(aq)} + \ce{Ag}^{+}{(aq)} + \ce{NO}_{3}^{-}{(aq)} {\rightarrow} \ce{AgCl}{(s)} + \ce{Na}^{+}{(aq)} + \ce{NO}_{3}^{-}{(aq)}[/latex]
the [latex]\ce{Ag}_{+}{(aq)}[/latex] and [latex]\ce{Cl}_{-}{(aq)}[/latex] ions become [latex]\ce{AgCl}{(s)}[/latex], but the [latex]\ce{Na}^{+}{(aq)}[/latex] ions and the [latex]\ce{NO}_{3}^{-}(aq)[/latex] ions stay as [latex]\ce{Na}{(aq)}[/latex] ions and [latex]\ce{NO}_{3}^{-}{(aq)}[/latex] ions. These two ions are examples of spectator ions, which are ions that do nothing in the overall course of a chemical reaction. They are present, but they do not participate in the overall chemistry. It is common to cancel spectator ions (something also done with algebraic quantities) on the opposite sides of a chemical equation:
[latex]\begin{multline*} \cancel{\ce{Na^+(aq)}}+\ce{Cl^-(aq)}+\ce{Ag^+(aq)}+\cancel{\ce{NO3^-(aq)}}\rightarrow \\ \ce{AgCl(s)}+\cancel{\ce{Na^+(aq)}}+\cancel{\ce{NO3^-(aq)}} \end{multline*}[/latex]
What remains when the spectator ions are removed is called the net ionic equation, which represents the actual chemical change occurring between the ionic compounds:
[latex]\ce{Cl}^{-}{(aq)} + \ce{Ag}^{+}{(aq)} {\rightarrow} \ce{AgCl}{(s)}[/latex]
It is important to reiterate that the spectator ions are still present in the solution, but they don’t experience any net chemical change, so they are not written in a net ionic equation.
Example 4.5.3
Problems
Write the net ionic equation for each chemical reaction.
- [latex]\ce{K}^{+}{(aq)} + \ce{Br}^{-}{(aq)} + \ce{Ag}^{+}{(aq)} + \ce{C}_{2}\ce{H}_{3}\ce{O}_{2}^{-}{(aq)} {\rightarrow} \ce{K}^{+}{(aq)} + \ce{C}_{2}\ce{H}_{3}\ce{O}_{2}^{-}{(aq)} + \ce{AgBr}{(s)}[/latex]
- [latex]\ce{Mg}^{2+}{(aq)} + \ce{SO}_{4}^{2-}{(aq)} + \ce{Ba}^{2+}{(aq)} + \ce{2NO}_{3}^{-}{(aq)} {\rightarrow} \ce{Mg}^{2+}{(aq)} + \ce{2NO}_{3}^{-}{(aq)} + \ce{BaSO}_{4}{(s)}[/latex]
Solution
- In the first equation, the K+(aq) and C2H3O2−(aq) ions are spectator ions, so they are cancelled:
[latex]\cancel{\ce{K^+(aq)}}+\ce{Br^-(aq) + Ag^+(aq)}+\cancel{\ce{C2H3O2^-(aq)}}\rightarrow \cancel{\ce{K^+(aq)}}+\cancel{\ce{C2H3O2^-(aq)}}+\ce{AgBr(s)}[/latex]
The net ionic equation is:[latex]\ce{Br}^{-}{(aq)} + \ce{Ag}^{+}{(aq)} {\rightarrow} \ce{AgBr}{(s)}[/latex]
- In the second equation, the Mg2+(aq) and NO3−(aq) ions are spectator ions, so they are cancelled:
[latex]\cancel{\ce{Mg^{2+}(aq)}} + \ce{SO4^{2-}(aq) + Ba^{2+}(aq)} + \cancel{\ce{2NO3^-(aq)}}\rightarrow \cancel{\ce{Mg^{2+}(aq)}}+\cancel{\ce{2NO3^-(aq)}}+\ce{BaSO4(s)}[/latex]
The net ionic equation is:[latex]\ce{SO}_{4}^{2-}{(aq)} + \ce{Ba}^{2+}{(aq)} → \ce{BaSO}_{4}{(s)}[/latex]
Test Yourself
Write the net ionic equation for:
[latex]\ce{CaCl}_{2}{(aq)} + \ce{Pb(NO_{3})}_{2}{(aq)} {\rightarrow} \ce{Ca(NO_{3})}_{2}{(aq)} + \ce{PbCl}_{2}{(s)}[/latex]
Answer
[latex]\ce{Pb}^{2+}{(aq)} + \ce{2Cl}^{-}{(aq)} {\rightarrow} \ce{PbCl}_{2}{(s)}[/latex]
Soluble and Insoluble Ionic Compounds
The concept of solubility versus insolubility in ionic compounds is a matter of degree. Some ionic compounds are very soluble, some are only moderately soluble, and some are soluble so little that they are considered insoluble. For most ionic compounds, there is also a limit to the amount of the compound that can be dissolved in a sample of water. For example, you can dissolve a maximum of [latex]36.0g[/latex] of [latex]\ce{NaCl}[/latex] in [latex]100g[/latex] of water at room temperature, but you can dissolve only [latex]0.00019g[/latex] of [latex]\ce{AgCl}[/latex] in [latex]100g[/latex] of water. We consider [latex]\ce{NaCl}[/latex] soluble but [latex]\ce{AgCl}[/latex] insoluble.
One place where solubility is important is in the tank-type water heater found in many homes. Domestic water frequently contains small amounts of dissolved ionic compounds, including calcium carbonate ([latex]\ce{CaCO_{3}}[/latex]). However, [latex]\ce{CaCO_{3}}[/latex] has the relatively unusual property of being less soluble in hot water than in cold water. So, as the water heater operates by heating water, [latex]\ce{CaCO_{3}}[/latex] can precipitate if there is enough of it in the water. This precipitate, called limescale, can also contain magnesium compounds, hydrogen carbonate compounds, and phosphate compounds. The problem is that too much limescale can impede the function of a water heater, requiring more energy to heat water to a specific temperature or even blocking water pipes into or out of the water heater, causing dysfunction.
Key Takeaways
- Ionic compounds that dissolve separately into individual ions.
- Complete ionic equations show dissolved ionic solids as separated ions.
- Net ionic equations show only the ions and other substances that change in a chemical reaction.
Exercises

