8.4 Naming and Drawing Hydrocarbons
Learning Objectives
- Name hydrocarbons using IUPAC nomenclature.
- Draw simple hydrocarbons.
Naming Hydrocarbons
Let’s apply what we have learned in section 8.3 to name an alkane.
Examples 8.4.1
Write the IUPAC name for the following molecule:
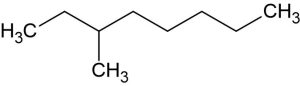
Step 1: Identify and name the parent carbon chain.
Find the longest continuous chain of carbon atoms in the molecule. This chain is the main carbon backbone of the alkane. The name of the alkane is based on the number of carbon atoms in the longest chain. Use appropriate prefixes to name the carbon chain. Then, add the suffix ‘ane’ to indicate membership in the alkane family.
For the example shown below, the longest carbon chain/parent chain is written in one line (highlighted in yellow). However, this might not be the case for some molecules. Then you might need to turn corners to search for it. As the given molecule contains 8 carbon atoms in its parent chain, it can be named an octane (‘Oct’ is the prefix, and ‘ane’ is the suffix).
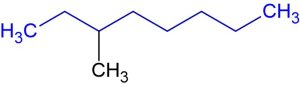
Step 2: Number the carbon atoms in the parent chain.
Number the carbon atoms in the main chain starting from the end nearest to the first substituent (if any). Assign the lowest number possible to the substituents.
The following molecule has one substituent. We should number the carbon atoms, giving the lowest possible number to the carbon atom attached to the substituent. When we start numbering from the left side, substituted carbon gets the third position. If we start numbering from the right side, substituted carbon gets the sixth position. Therefore, we start numbering from the left as shown in the following figure.
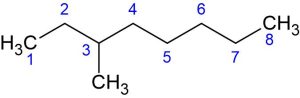
Step 3: Name and locate substituents.
If there are any substituents (groups attached to the main chain), name them using prefixes like methyl, ethyl, propyl, etc. or functional group name, for instance, hydroxy (for [latex]\ce{OH^{-}}[/latex], chloro ([latex]\ce{Cl^{-}}[/latex] etc.
Specify the location of each substituent by indicating the number of the carbon atom to which it is attached.
The following molecule has a methyl substituent [latex]\ce{CH_{3}}[/latex] attached to the third carbon in the parent chain.
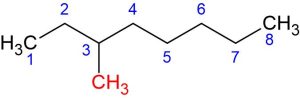
Sometimes, there can be more substituents attached to different carbons or the same carbon. For instance, consider the following structure:
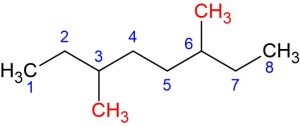
This structure has two methyl groups attached to the third and sixth carbons.
Step 4: Complete the name.
Write the complete name in one word, using hyphens to separate words and commas to separate numbers. Alphabetise the substituent names when there are multiple substituents. Use appropriate prefixes, such as di, tri, etc., if the same substituent is present more than once.
The IUPAC name of the following molecule would be 3-Methyloctane:

The IUPAC name of the following molecule would be 3,6-Dimethyloctane:

Naming Alkenes and Alkynes
When faced with a structure containing a functional group, such as an alkene, the name of the related alkene can be a good starting point. Most elements of the name will be the same, with the exception that the identity and location of the functional group itself need to be conveyed somehow. For the alkenes, the suffix used is no longer ‘ane’ but is now ‘one.’ The location of the double bond is identified with a number. Count a parent chain that includes the alkene, counting from the end of the chain with the lowest possible number assignment given to the double bond. Then, use the number of the carbon where the double bond is first encountered as the location indicator. Current IUPAC rules put the number immediately before the ‘ene’ suffix, but name changes are sometimes accepted rather slowly; it remains very common to see this number earlier in the name.
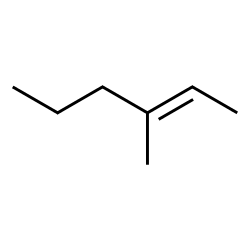
Acceptable names for the above molecule ( Figure 8.4.1) include 3-methylhex-2-ene and 3-methyl-2-hexene. IUPAC rules encourage placing the location identifier close to the feature at that location. The first name follows IUPAC rules to the letter. However these names can seem awkward even to chemists, and the second form is used frequently.
Other aspects of naming alkenes are identical to the process used for alkanes: the parent chain is indicated by the base name, and the branches are numbered and named just as they are for alkanes.
Alkynes are named similarly to alkenes.
Summary of Naming Rules for Alkenes and Alkynes
- The longest chain of carbon atoms containing the double or triple bond is considered the parent chain. It is named using the same stem as the alkane having the same number of carbon atoms but ends in –ene to identify it as an alkene. Thus, the compound CH2=CHCH3 is propene. Alkynes are similarly indicated, using the suffix -yne.
- If there are four or more carbon atoms in a chain, we must indicate the position of the double or triple bond. The carbon atoms are numbered so that the first of the two that are doubly or triply bonded is given the lower of the two possible numbers. The compound CH3CH=CHCH2CH3, for example, has a double bond between the second and third carbon atoms. Its name is 2-pentene (not 3-pentene).
-
Substituent groups are named as with alkanes, and their position is indicated by a number. Thus, Figure 8.4.2 is 5-methyl-2-hexene. Note that the numbering of the parent chain is always done in such a way as to give the double bond the lowest number, even if that causes a substituent to have a higher number. The double bond always has priority in numbering.
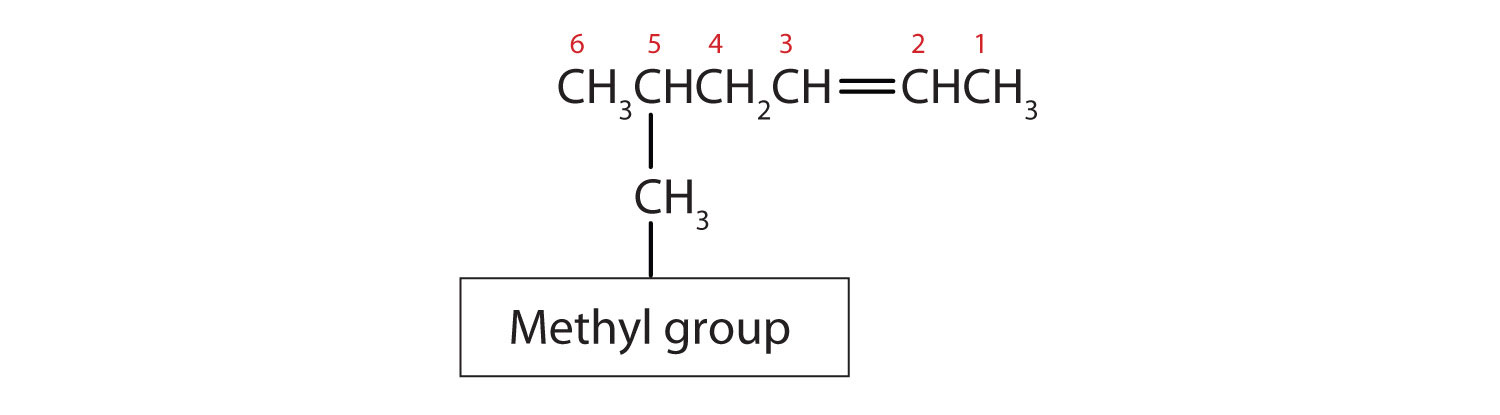
Figure 8.4.2 Structure of 5-methyl-2-hexene
Examples 8.4.2
Problem
Name each of the following compounds.
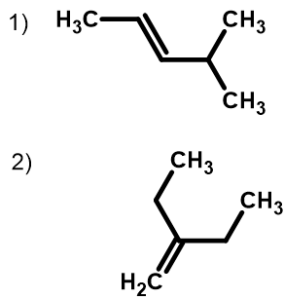
Solution
- The longest chain containing the double bond has five carbon atoms, so the compound is a pentene (rule 1). To give the first carbon atom of the double bond the lowest number (rule 2), we number from the left, so the compound is a 2-pentene. There is a methyl group on the fourth carbon atom (rule 3), so the compound’s name is 4-methyl-2-pentene.
- The longest chain containing the double bond has four carbon atoms, so the parent compound is a butene (rule 1). (The longest chain overall has five carbon atoms, but it does not contain the double bond, so the parent name is not pentene.) To give the first carbon atom of the double bond the lowest number (rule 2), we number from the left, so the compound is a 1-butene. There is an ethyl group on the second carbon atom (rule 3), so the compound’s name is 2-ethyl-1-butene.
Test Yourself
Name the following compound:
[latex]\ce{CH_{3}CH_{2}CH_{2}CH_{2}CH_{2}CH=CHCH_{3}}[/latex]
Answer
2-Octene
Drawing Hydrocarbons
Let’s apply what we have learned about drawing organic molecules in section 8.2 to draw hydrocarbons.
Examples 8.4.3
Draw the structure for each compound.
- 3-methyl-2-pentene
- cyclohexene
Solution
-
First, write the parent chain of five carbon atoms: C–C–C–C–C. Then add the double bond between the second and third carbon atoms:

Now, place the methyl group on the third carbon atom and add enough hydrogen atoms to give each carbon atom a total of four bonds:
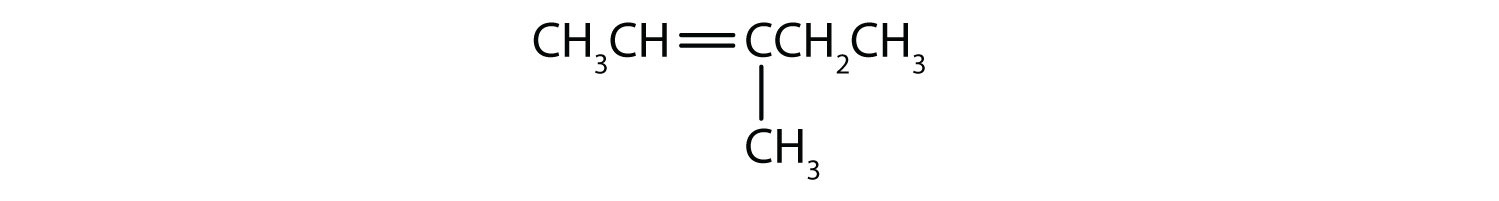
-
Just as there are cycloalkanes, there are cycloalkenes. These compounds are named like alkenes but with the prefix cyclo– attached to the beginning of the parent alkene name. First, consider what each of the three parts of the name means. Cyclo means a ring compound, hex means 6 carbon atoms, and –ene means a double bond:
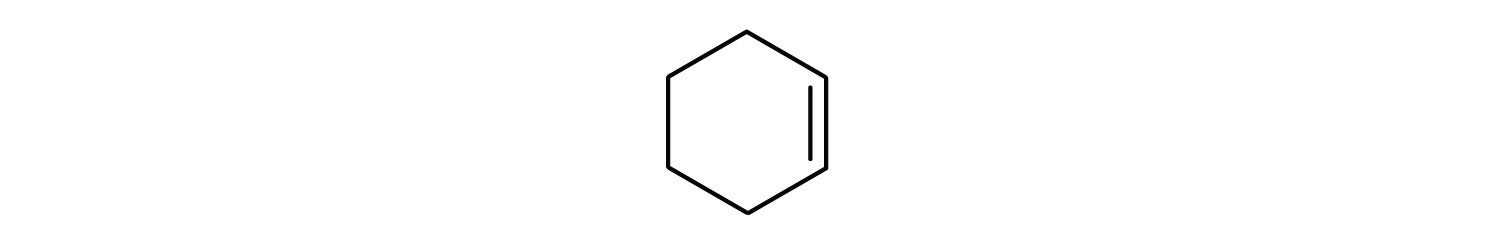
Key Takeaways
- All hydrocarbons are named in a similar way; however, the position of double and triple bonds present in alkenes and alkynes, respectively, need to be stated in the name.
- IUPAC name of an organic molecule provides sufficient information to draw the respective molecule.
- Hydrocarbons can be drawn using line-bond and structural formulas.
Exercises

