7.2 Thermochemistry Essentials
Learning Objectives
- Define how temperature and heat are understood on the molecular level by Collision Theory.
- Learn about the absolute scale of the Kelvin and its relationship to degrees Celsius.
- Define the three types of heat transfer: conduction, convection and radiation.
- Define the three types of thermodynamic systems: open, closed and isolated, and the allowed transfer of matter and energy in each.
Video 7.2.1: Collision Theory and Rates of Reaction. An introduction into Collision Theory. Video attribution: “Collision Theory and Rates of Reaction” by RMIT Library. © 2024 RMIT Library, licensed under CC BY-NC-SA 4.0.
Collision Theory
To understand thermochemistry, we must first talk about the basics of collision theory. Collision theory is a chemical model which applies physics principles to understand the mechanisms of reactions. Let’s consider the formation of water: [latex]\ce{2H2 + O2 -> 2H2O}[/latex]. Under this model, we will represent all molecules as balls, as demonstrated in Figure 7.2.1.
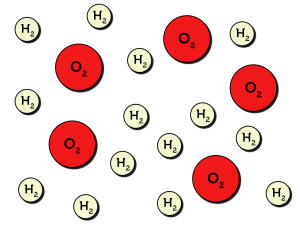
From your own experience (in the lab or cooking on a stove), you may know that the temperature of a system correlates to the rate of a reaction. According to collision theory, temperature is directly related to the kinetic energy of molecules. As temperature rises, the speed at which molecules move increases (see Figure 7.2.2).
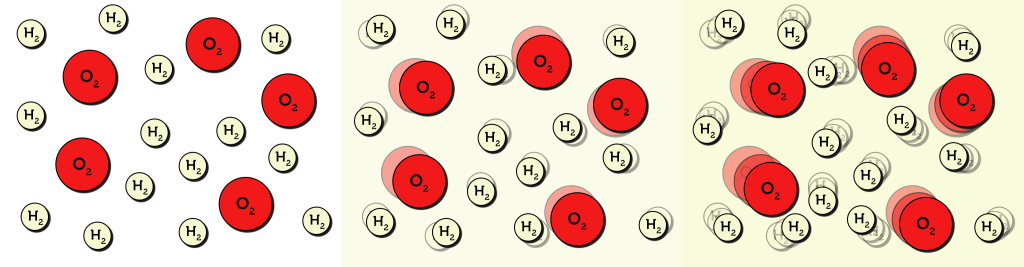
With molecules moving faster, they are more likely to collide with each other with enough energy, causing a reaction to take place. Higher temperatures allow more successful collisions to occur more often, increasing the reaction rate.
The Kelvin
With this understanding, we must revisit how temperature is traditionally measured. The majority of the world utilises degrees Celsius (°C) – a system based on the freezing (0°C) and boiling (100°C) point of water. However, a reading of 0°C does not mean that no energy exists within a system; a lower temperature, such as -5°C, can still be achieved. For our energy calculations, an absolute value is needed: the Kelvin (K). The conversion from degrees Celsius to Kelvin is as follows:
[latex]K = °C + 273.15[/latex]
Under the Kelvin system, a value of 0 K represents that a molecule has no kinetic energy and is completely still. This is known as absolute zero[1]. Energy calculations within science are generally performed in Kelvin for this reason.
Conduction
With our understanding of temperature being a measure of kinetic energy, we can appreciate how heat occurs. Heat is the transfer of energy from one body to another. This can only occur when a temperature difference is present. A hotter body will impart its energy onto a colder body until thermodynamic equilibrium is established. As heat is transferred, the hotter body will cool down as it loses its kinetic energy, while the colder body will begin to rise in temperature (see Figure 7.2.3).
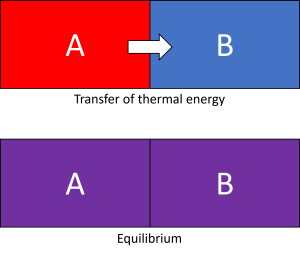

Therefore, what we consider to be hot or cold isn’t due to the temperature itself – but the temperature difference. Something appears to feel cold because our hands are hotter than it is (see Figure 7.2.4), and vice versa. Our morning coffee on a particularly chilly day might appear to be warmer than usual because our bodies are colder than normal. This is also why some people find it hard to check their own temperature by touch when feverish – if their whole body is getting hotter, nothing will feel out of the ordinary even though body temperatures have risen.
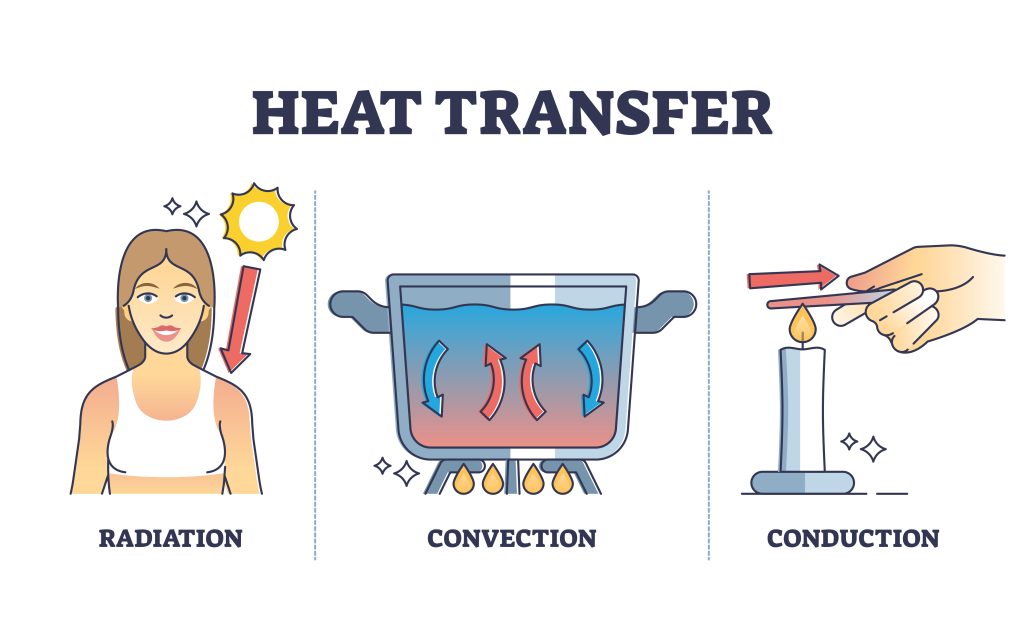
What we have just described is conduction: the movement of thermal energy between two bodies in physical contact. It is the simplest of three main ways to transfer energy. From cooking a steak on a cast-iron pan to the cold feeling of placing an icepack on your body – conduction is the most direct form of heat transfer.
Convection
Thermal transfer is, however, not limited to solid objects. When a pot of water is heated up, how does that thermal energy spread itself around the container?
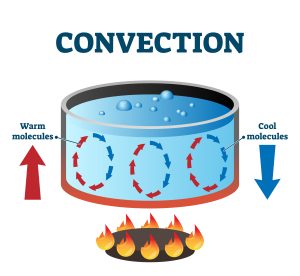
All fluids exhibit convection. As liquids or gasses are heated, hotter areas become less dense and begin to rise, allowing colder molecules to replace them and be heated themselves. This movement creates a convection current (see Figure 7.2.5).
An air-fryer or convection oven uses this concept to its advantage. By heating up food through a convection current of air, a more uniform temperature can be achieved. While a frying pan can only heat food from one side at a time, a convection oven can heat from everywhere at once. To assist in this movement, fans are used to improve efficiency – allowing cooking temperatures to be reduced in fan-forced systems.
Radiation
So far, the models we have looked at rely on matter to be present to transfer thermal energy. However, there is no matter in space, so how does the sun transfer heat to our planet?
The sun produces a wide range of electromagnetic radiation. Our eyes are able to detect only a small range of wavelengths (known as the visible spectrum; see Figure 7.2.5) – but a variety of other wavelengths are emitted.
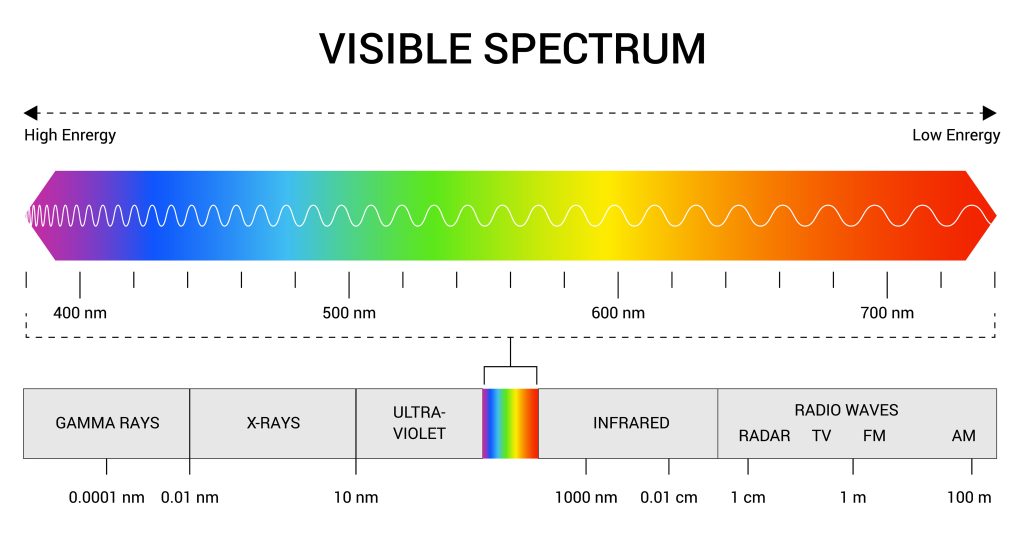
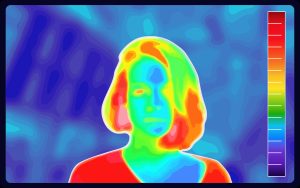
Infrared is the most common form of radiation generating heat. All objects with heat emit radiation, although hotter bodies manifest higher energy waves (see Figure 7.2.6). These wavelengths collide with molecules – causing some kinetic energy to be imparted. With more kinetic energy, a higher temperature is achieved and the objected is heated.
As such, thermal radiation requires no physical matter between the source and a target of interest to transfer energy. In fact, all forms of electromagnetic radiation do not require a medium to travel through, explaining why the sun can impart light and energy onto the Earth even through the vacuum of space.
Systems of Heat
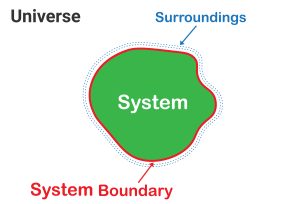
With all 3 forms of heat transfer discussed (see Figure 7.2.7), we can understand how different thermal systems operate. In this context, a system refers to the part of the universe under study. Between the universe and the system is its direct surroundings – which can exchange heat and matter depending on the system (see Figure 7.2.8). The type of exchange depends on the system utilised. We observe three main types: open, closed, and isolated (see Figure 7.2.9).
An open system is one where both matter and energy can be exchanged with the environment. A boiling pot of water can be considered an open system: heat can radiate and conduct out to the surrounding environment, and water (in the form of steam) can escape. This is an important system for distillation and extraction methods. A closed system limits the transfer of matter but allows the transmittance of heat. By placing a lid on our pot, we are preventing matter from escaping the system as heat. We can, however, still heat up or cool down our system. An isolated system prevents both mass and heat from escaping into the surroundings. Heat transfer via convection can be limited through the implementation of a vacuum surrounding the system, while a reflective surface limits energy loss through radiation.
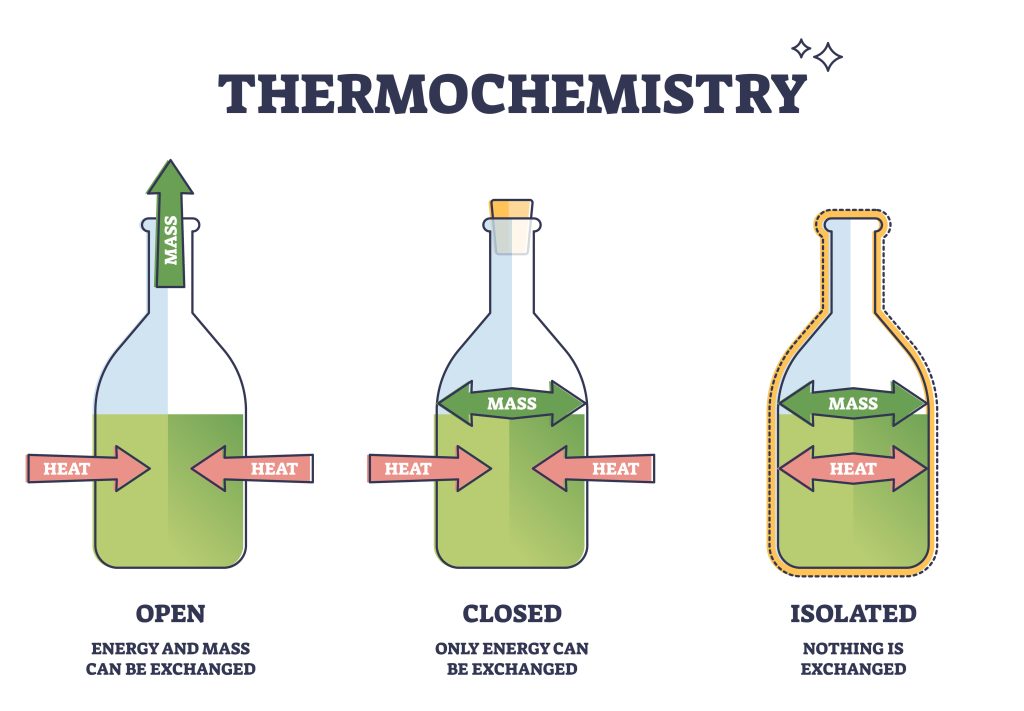
Energy studies performed within thermochemistry can involve all of these different types of systems. Understanding what flows from a system to its surroundings and the larger universe, alongside accounting for it, allows us to perform many different thermochemical calculations and experiments.
Key Takeaways
- Temperature is a measure of the kinetic energy of molecules. As temperature raises, the speed which molecules move does as well.
- In science, the Kelvin is the preferred measure of temperature when performing calculations. 0 K is known as absolute zero.
- All bodies in contact will attempt to reach thermodynamic equilibrium.
- There are three types of heat transfer: conduction (physical contact); convection (fluid currents); and radiation (electromagnetic waves).
- There are three types of systems: open (matter and energy exchange); closed (only energy exchanged), and isolated (no transfer)
Exercises
Media Attributions
- child holding ice cubes – winter © BarbaraKrupa - stock.adobe.com is licensed under a All Rights Reserved license
- Convection currents vector illustration labeled diagram © VectorMine - stock.adobe.com is licensed under a All Rights Reserved license
- Spectrum wavelength. Visible spectrum color range. Educational physics light line. Light wave frequency. Wavelengths of the visible part of the spectrum for human eyes © designer_things - stock.adobe.com is licensed under a All Rights Reserved license
- Vector graphic of Thermographic image of a woman face showing different temperatures in a range of colors from blue showing cold to red showing hot. Medical thermal imaging of human female face. © Cipta - stock.adobe.com is licensed under a All Rights Reserved license
- Heat transfer types with radiation, convection and conduction types outline diagram. Labeled educational scheme with thermal energy exchange methods vector illustration. Hot temperature sources list. © VectorMine - stock.adobe.com is licensed under a All Rights Reserved license
- Thermodynamic system, boundary, system and surroundings © Reuel Sa - stock.adobe.com
- Thermochemistry heat exchange as thermodynamics study brunch outline diagram. Labeled educational open, closed and isolated systems with mass and heat physical forces type scheme vector illustration. © VectorMine - stock.adobe.com is licensed under a All Rights Reserved license
- . Absolute zero is a theoretical concept and cannot physically be achieved. ↵
A chemical model used to predict the rates of reactions with respect to a molecules orientation and energy.
The part of the universe that is under study.
A measure of the average amount of kinetic energy a system contains.
Energy due to motion.
Common unit to express temperature - based on the freezing and boiling points of water at SLC.
The fundamental unit of temperature in SI.
The minimum possible temperature, labelled 0 K (zero kelvins).
The transfer of energy from one body to another due to a difference in temperature.
The point at which two bodies in contact reach the same temperature as one another.
The transfer of thermal energy due to the physical contact of two bodies.
A substance that has no fixed shape; a liquid or gas.
Temperature transfer due to the movement of hot and cold molecules within a fluid.
Temperature transfer due to electromagnetic wavelengths exciting molecules.
The environment which encompasses all matter and energy to ever exist.
Area of space between the system and the universe of a thermodynamic study.
System which exchanges both matter and energy with its environment.
System which only exchanges energy with its environment
A system that does not allow a transfer of energy or matter into or out of itself.

