6.6 Buffers
Learning Objectives
- Define a buffer.
- Correctly identify the two components of a buffer.
Weak acids are relatively common, even in the foods we eat. But we occasionally encounter a strong acid or base, such as stomach acid, which has a strongly acidic pH of 1.7. By definition, strong acids and bases can produce a relatively large amount of [latex]\ce{H^{+}}[/latex] or [latex]\ce{OH^{-}}[/latex] ions and consequently have marked chemical activities. In addition, very small amounts of strong acids and bases can change the pH of a solution very quickly. If 1 mL of stomach acid [approximated as [latex]{0.1 M}\ce{HCl}{(aq)}[/latex]] were added to the bloodstream and no correcting mechanism were present, the pH of the blood would decrease from about 7.4 to about 4.7 — a pH that is not conducive to continued living. Fortunately, the body has a mechanism for minimising such dramatic pH changes.
This mechanism involves a buffer, a solution that resists dramatic changes in pH. Buffers do so by being composed of certain pairs of solutes: either a weak acid plus a salt derived from that weak acid or a weak base plus a salt of that weak base. For example, a buffer can be composed of dissolved [latex]\ce{HC_{2}H_{3}O_{2}}[/latex] (a weak acid) and [latex]\ce{NaC_{2}H_{3}O_{2}}[/latex] (the salt derived from that weak acid). Another example of a buffer is a solution containing [latex]\ce{NH_{3}}[/latex] (a weak base) and [latex]\ce{NH_{4}Cl}[/latex] (a salt derived from that weak base).
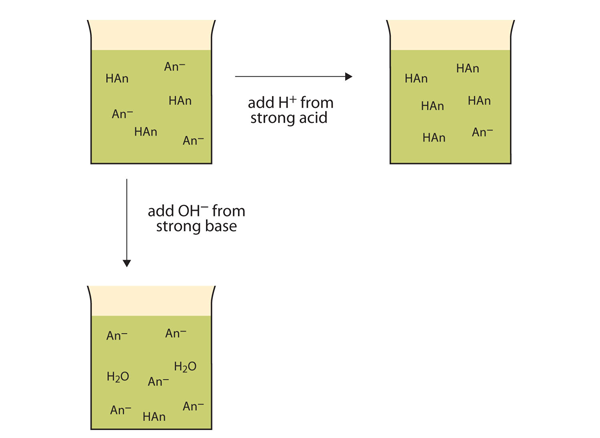
Let us use an [latex]\ce{HC_{2}H_{3}O_{2}}[/latex] / [latex]\ce{NaC_{2}H_{3}O_{2}}[/latex] buffer to demonstrate how buffers work. If a strong base — a source of [latex]\ce{OH^{-}}{(aq)}[/latex] ions — is added to the buffer solution, those [latex]\ce{OH^{-}}[/latex] ions will react with the [latex]\ce{HC_{2}H_{3}O_{2}}[/latex] in an acid-base reaction, as shown below:
[latex]\ce{HC2H3O2(aq)}+\ce{OH-(aq)}\rightarrow \ce{H2O(\ell)}+\ce{C2H3O2-(aq)}[/latex]
Rather than changing the pH dramatically by making the solution basic, the added [latex]\ce{OH^{-}}[/latex] ions react to make [latex]\ce{H_{2}O}[/latex], so the pH does not change much.
If a strong acid — a source of [latex]\ce{H^{+}}[/latex] ions — is added to the buffer solution, the [latex]\ce{H^{+}}[/latex]ions will react with the anion from the salt. Because [latex]\ce{HC_{2}H_{3}O_{2}}[/latex] is a weak acid, it is not ionised much. This means that if lots of [latex]\ce{H^{+}}[/latex] ions and [latex]\ce{C_{2}H_{3}O_{2}^{-}}[/latex] ions are present in the same solution, they will come together to make [latex]\ce{HC_{2}H_{3}O_{2}}[/latex], as shown in the following equation:
[latex]\ce{H+(aq)}+\ce{C2H3O2-(aq)}\rightarrow \ce{HC2H3O2(aq)}[/latex]
Rather than changing the pH dramatically and making the solution acidic, the added [latex]\ce{H^{+}}[/latex] ions react to make molecules of a weak acid. Figure 6.6.1 "The Actions of Buffers" illustrates both actions of a buffer.
Buffers made from weak bases and the salts of weak bases act similarly. For example, in a buffer containing [latex]\ce{NH_{3}}[/latex] and [latex]\ce{NH_{4}Cl}[/latex], [latex]\ce{NH_{3}}[/latex] molecules can react with any excess [latex]\ce{H^{+}}[/latex] ions introduced by strong acids, as shown below:
[latex]\ce{NH3(aq)}+\ce{H+(aq)}\rightarrow \ce{NH4+(aq)}[/latex]
While the [latex]\ce{NH_{4}^{+}}{(aq)}[/latex] ion can react with any [latex]\ce{OH^{-}}[/latex] ions introduced by strong bases, as can be seen in the following equation:
[latex]\ce{NH4+(aq)}+\ce{OH-(aq)}\rightarrow \ce{NH3(aq)}+\ce{H2O(\ell)}[/latex]
Example 6.6.1
Problem
Which combinations of compounds can make a buffer solution?
- [latex]\ce{HCHO_{2}}[/latex] and [latex]\ce{NaCHO_{2}}[/latex]
- [latex]\ce{HCl}[/latex] and [latex]\ce{NaCl}[/latex]
- [latex]\ce{CH_{3}NH_{2}}[/latex] and [latex]\ce{CH_{3}NH_{3}Cl}[/latex]
- [latex]\ce{NH_{3}}[/latex] and [latex]\ce{NaOH}[/latex]
Solution
- [latex]\ce{HCHO_{2}}[/latex] is formic acid, a weak acid, while [latex]\ce{NaCHO_{2}}[/latex] is the salt made from the anion of the weak acid (the formate ion [latex]\ce{CHO_{2}^{-}}[/latex]). The combination of these two solutes would make a buffer solution.
- [latex]\ce{HCl}[/latex] is a strong acid, not a weak acid, so the combination of these two solutes would not make a buffer solution.
- [latex]\ce{CH_{3}NH_{2}}[/latex] is methylamine, which is like [latex]\ce{NH_{3}}[/latex], with one of its [latex]\ce{H}[/latex] atoms substituted with a [latex]\ce{CH_{3}}[/latex] group. It is a weak base. The compound [latex]\ce{CH_{3}NH_{3}Cl}[/latex] is a salt made from that weak base, so the combination of these two solutes would make a buffer solution.
- [latex]\ce{NH_{3}}[/latex] is a weak base, but [latex]\ce{NaOH}[/latex] is a strong base. The combination of these two solutes would not make a buffer solution.
Test Yourself
Which combinations of compounds can make a buffer solution?
- [latex]\ce{NaHCO_{3}}[/latex] and [latex]\ce{NaCl}[/latex]
- [latex]\ce{H_{3}PO_{4}}[/latex] and [latex]\ce{NaH_{2}PO_{4}}[/latex]
- [latex]\ce{NH_{3}}[/latex] and [latex]\ce{(NH_{4})_{3}PO_{4}}[/latex]
- [latex]\ce{NaOH}[/latex] and [latex]\ce{NaCl}[/latex]
Answers
- no
- yes
- yes
- no
Buffers work well only for limited amounts of added strong acid or base. Once either solute is completely reacted, the solution is no longer a buffer, and rapid changes in pH may occur. We say that a buffer has a certain capacity. Buffers that have more solute dissolved in them to start with have larger capacities, as might be expected.
Human blood has a buffering system to minimise extreme changes in pH. One buffer in blood is based on the presence of [latex]\ce{HCO_{3}^{-}}[/latex] and [latex]\ce{H_{2}CO_{3}}[/latex] (the second compound is another way to write [latex]\ce{CO_{2}}{(aq)}[/latex]). With this buffer present, even if some stomach acid were to find its way directly into the bloodstream, the change in the pH of blood would be minimal. Inside many of the body’s cells, there is a buffering system based on phosphate ions.
The Acid That Eases Pain
Let’s take a look at an acid that is probably the most common medicine: acetylsalicylic acid, also known as aspirin. Aspirin is well known as a pain reliever and antipyretic (fever reducer).
The structure of aspirin is shown in the accompanying figure. The acid part is circled; it is the hydrogen atom in that part that can be donated as aspirin acts as a Brønsted-Lowry acid. Acetylsalicylic acid is a weak acid. However, it is still an acid, and given that some people consume relatively large amounts of aspirin daily, its acidic nature can cause problems in the stomach lining despite the stomach’s defences against its own stomach acid.
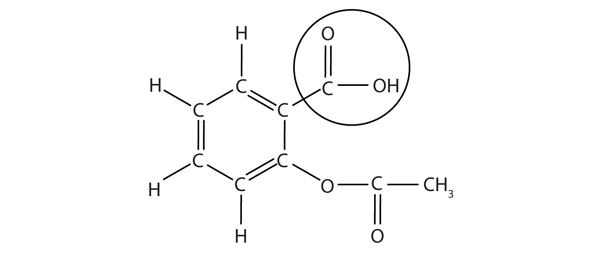
Because the acid properties of aspirin may be problematic, many aspirin brands offer a “buffered aspirin” form of the medicine. In these cases, the aspirin also contains a buffering agent — usually [latex]\ce{MgO}[/latex] — that regulates the acidity of the aspirin to minimise its acidic side effects.
Key Takeaways
- A buffer is a solution that resists sudden changes in pH.
Exercises
A Brønsted-Lowry acid is any species that can donate a proton to another molecule.

