1.1 States of Matter
Learning Objectives
- Learn the basic terms used to describe matter.
What is Matter?
Matter encompasses anything that has mass and occupies space. This includes things that can be seen (all living and non-living, natural and synthetic things) and cannot be seen by the naked eye, like air and microorganisms. For instance, a book, a computer, food and dirt are all examples of matter. Sometimes, the matter may be difficult to identify. For example, air is matter, but because it is so thin compared to other matter (like books, computers, food, and dirt), we sometimes forget that air has mass and takes up space. In contrast, immaterial concepts and things like ideas, emotions and hopes do not qualify as matter.
Properties of Matter
To understand matter and how it changes, we need to be able to describe matter. There are two basic ways to describe matter: physical properties and chemical properties. Physical properties are characteristics that describe matter as it exists, such as shape, colour, size and temperature. An important physical property is the phase (or state) of matter. The three fundamental phases of matter are solid, liquid, and gas. If we take water as an example, water can exist in all physical states: solid-ice cubes, liquid-liquid water, and gas-water vapour. Let’s see how each of these states differ from one another.
Solid phase: the particles in a solid are packed tightly together, preventing the movement of particles and locking them into position (as shown in Figure 1.1.1), so that they cannot pass each other. Solids have a definite shape and a definite volume. Ice cubes are an example of a solid.

Liquid phase: The particles in a liquid are close together. However, the distance between the particles allows them to move past each other. Liquids take on the shape of the container, as they have an indefinite shape. Liquids have a definite volume. Think about a glass of water. The water takes the shape of the glass it is placed in, and we can define its volume, for instance, half a cup and a quarter cup.

Gaseous Phase: the particles in a gas are separated and lack structure (Figure 1.1.3). Gas takes the shape of a container as gas has an indefinite shape. Gas has an indefinite volume—for example, water vapour.

Chemical properties define how matter behaves in the presence of other matter in terms of its ability to undergo chemical transformations and change its form through chemical reactions. Understanding these properties is crucial for predicting and explaining the behaviour of matter in various chemical contexts. Chemical properties can not be observed or measured without changing the chemical identity of the substance. Take, for instance, the rusting of iron objects after exposure to moist air for a prolonged period (Figure 1.1.4). Here, the matter is iron, which has changed to iron oxide (rust). This means the initial chemical composition has changed while observing the chemical property, which is called corrosion [1]. So, we say iron has the chemical property of corrosion. Another example is when wood burns, it changes to ashes. We say wood has the chemical property of flammability [2].

Changes in the Matter
If matter always stayed the same, chemistry would be rather boring. Fortunately, a major part of chemistry involves change. A physical change occurs when a sample of matter changes one or more of its physical properties. For example, a solid may melt (Figure 1.1.7 ), or alcohol in a thermometer may change volume as the temperature changes. A physical change does not affect the chemical composition of matter. A chemical change is the process of demonstrating a chemical property, such as the burning match in Figure 1.1.5 “Chemical Properties”. As the matter in the match burns, its chemical composition changes and new forms of matter with new physical properties are created. Note that chemical changes are frequently accompanied by physical changes, as the new matter will likely have different physical properties from the original matter.
Physical Change: Phase Change of Matter
A phase change refers to the transformation of a substance from one state of matter to another. The three primary states of matter are solid, liquid, and gas. Variations in temperature or pressure typically cause phase changes between these states.
Water is an excellent example to illustrate phase changes because it can exist in all three states.
- Melting (Solid to Liquid): When you add heat to ice (solid water) at its melting point (0 degrees Celsius or 32 degrees Fahrenheit at standard atmospheric pressure), it absorbs energy and undergoes a phase change into liquid water.
- Freezing (Liquid to Solid): The opposite of melting; freezing occurs when you remove heat from liquid water. The water molecules lose energy and arrange themselves into a more ordered, solid structure (ice).
- Vaporisation (Liquid to Gas): Vaporisation is the process by which a liquid turns into a gas. It can occur in two forms: boiling and evaporation. Boiling happens at the liquid’s boiling point. For water, boiling occurs at 100 degrees Celsius or 212 degrees Fahrenheit at standard atmospheric pressure, while evaporation can occur at any temperature.
- Condensation (Gas to Liquid): Condensation is the reverse of vaporisation. It happens when a gas loses heat and transforms into a liquid. This occurs, for example, when water vapour in the air cools and forms dew on a cold surface.
- Sublimation (Solid to Gas): Sublimation is the process by which a substance transitions directly from a solid to a gas without passing through the liquid phase. An example of this is dry ice (solid carbon dioxide) turning into carbon dioxide gas.
- Deposition (Gas to Solid): Deposition is the reverse of sublimation. It occurs when a gas transforms directly into a solid without going through the liquid phase. Frost forming on a cold surface is an example of deposition.
These phase changes are governed by the principles of thermodynamics, which describe the relationship between heat, energy, and matter (you will learn the basics of thermodynamics in chapter 7). The energy involved in these processes is typically associated with breaking or forming intermolecular forces between the particles of the substance.

Examples 1.1.1
Problems
Describe each process as a physical change or a chemical change.
- Water in the air turns into snow.
- A person’s hair is cut.
- Bread dough becomes fresh bread in an oven.
Solutions
- Because the water is going from a gas phase to a solid phase, this is a physical change.
- Your long hair is being shortened. This is a physical change.
- Because of the oven’s temperature, chemical changes are occurring in the bread dough to make fresh bread. These are chemical changes. (In fact, a lot of cooking involves chemical changes.)
Test Yourself
Identify each process as a physical change or a chemical change.
- A fire is raging in a fireplace.
- Water is warmed to make a cup of coffee.
Answers
- Chemical change.
- Physical change.
Classification of Matter
A sample of matter that has the same physical and chemical properties throughout is called a substance. Sometimes, the phrase pure substance is used, but the word pure isn’t needed. This definition of substance is an example of how chemistry has a specific definition for a word that has been used in everyday language with a different, more vague definition. Here, we will use the term substance with its strict chemical definition.
Chemistry recognises two different types of substances: elements and compounds. An element is the simplest type of chemical substance; it cannot be broken down into simpler chemical substances by ordinary chemical means. There are about 115 elements known to science, of which 80 are stable. The other elements are radioactive. Each element has its own unique set of physical and chemical properties. Examples of elements include iron, carbon, and gold.
A compound is a combination of more than one element. The physical and chemical properties of a compound are different from the physical and chemical properties of its constituent elements; that is, compounds behave as a substance that is completely different from the individual elements they are made from. There are over 50 million compounds known, and more are being discovered daily. Examples of compounds include water, penicillin, and sodium chloride (the chemical name for common table salt).
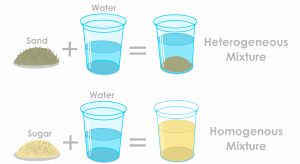
Elements and compounds are not the only ways matter can exist. We frequently encounter objects that are physical combinations of more than one element or compound. Physical combinations of more than one substance are called mixtures. There are two types of mixtures. A heterogeneous mixture is a mixture composed of two or more substances. It is easy to tell, sometimes by the naked eye, that more than one substance is present. A homogeneous mixture is a combination of two or more substances that is so intimately mixed that the mixture behaves as a single substance. Another word for a homogeneous mixture is a solution. Thus, a combination of salt and steel wool is a heterogeneous mixture because it is easy to see which particles of the matter are salt crystals and which are steel wool. On the other hand, if you take salt crystals and dissolve them in water, it is very difficult to tell that you have more than one substance present just by looking—even if you use a powerful microscope. The salt dissolved in water is a homogeneous mixture or a solution. Figure 1.1.6 displays examples of homogeneous and heterogenous mixtures.
There are other descriptors that we can use to describe matter, especially elements. We can usually divide elements into metals and nonmetals, and each set shares certain (but not always all) properties. A metal is an element that is solid at room temperature (although mercury is a well-known exception), is shiny and silvery, conducts electricity and heat well, can be pounded into thin sheets (a property called malleability), and can be drawn into thin wires (a property called ductility). A nonmetal is an element that is brittle when solid, does not conduct electricity or heat very well, and cannot be made into thin sheets or wires. Nonmetals also exist in a variety of phases and colours at room temperature. Some elements have properties of both metals and nonmetals and are called metalloids. We will see later how these descriptions can be easily assigned to various elements.
Examples 1.1.2
Problems
Identify the following combinations as heterogeneous mixtures or homogenous mixtures.
- Soda water (carbon dioxide is dissolved in water).
- A mixture of iron metal filings and sulphur powder (both iron and sulphur are elements).
Solutions
- Because carbon dioxide is dissolved in water, we can infer from the behaviour of salt crystals dissolved in water that carbon dioxide dissolved in water is (also) a homogeneous mixture.
- Assuming that the iron and sulphur are simply mixed together, it should be easy to see what is iron and what is sulphur, so this is a heterogeneous mixture.
Test Yourself
Are the following combinations homogeneous mixtures or heterogeneous mixtures?
- The human body
- An amalgam, a combination of some other metals dissolved in a small amount of mercury
Answers
- Heterogeneous mixture.
- Homogeneous mixture.
Watch the following simulation about the state of matter and phase changes.
Key Takeaways
- Matter is anything that has mass and takes up space.
- Matter can be described in terms of physical properties and chemical properties.
- Physical properties and chemical properties of matter can change.
- Matter is composed of elements and compounds.
- Combinations of different substances are called mixtures.
- Elements can be described as metals, nonmetals, and semimetals.
Exercises
Media Attributions
- Old rust-covered motorcycle in an abandoned building. AI-generated. © Wirestock - stock.adobe.com
- Homogeneous, heterogeneous mixtures. Salt or sugar solution, sea. Sand depression with water in glass. Solute, solvent molecules. Solid, liquid mix. Chemistry with explanations, Illustration Vector © LuckySoul - stock.adobe.com
Anything that has mass and takes up space.
Matter that has the same physical and chemical properties throughout.
A substance that cannot be broken down into simpler chemical substances by ordinary chemical means.
A combination of more than one element.

