2.4 Periodic Trends
Learning Objectives
- Understand how certain properties of atoms vary based on their relative position on the periodic table.
Arranging the periodic table by electron configuration allows us to observe trends within elements across groups and periods. Let us discuss some important chemical properties which can be observed.
Atomic radii
The atomic radius is an indication of the size of an atom. While atoms don’t have a size in the traditional sense (due to the fluctuating position of electrons), they behave as if they have a radius, particularly with reference to bonding. Atomic radii increase from the top to the bottom of a group of the periodic table. As [latex]n[/latex] increases when proceeding down a group, orbitals become larger, increasing atomic radii (see Figure 2.4.1).
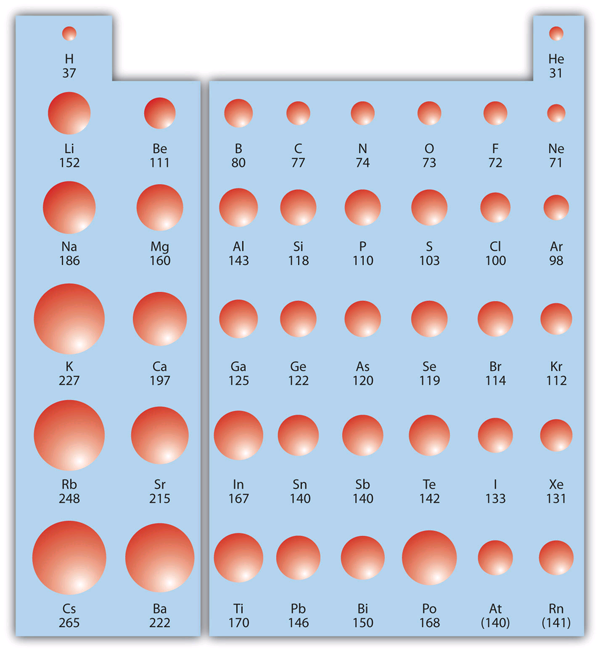
Atoms as Balls: Why the approximation?
While atoms aren’t small balls floating around in space, it can be handy for us to treat them as such! In constructing 3D models of compounds, the relative sizes of molecules should be taken into consideration. You don’t need to know the specifics, but should be able to take an educated guess at what is bigger on the periodic table. Consider [latex]H_2O[/latex], how big are the hydrogen atoms compared to the oxygen?
In chapter 7.2, Thermochemistry Essentials, we will talk about collision theory — in which atoms and molecules are seen as whole spherical objects that can collide with one another. In tertiary studies, we can mathematically work out the chances of a collision occurring through the size of the cross-section of a molecule. It is much easier to determine the probability of a sphere colliding than a complex object. For simple compounds, such as diatomic molecules comprised of a large atom and a relatively small atom, this approximation works.
Naturally, simplifications like this always bring about inaccuracy in our answers — however these are acceptable at this stage in your chemical career!
Ionisation energy
The first ionisation energy is the minimum energy required to remove one electron from a neutral atom. The second and third ionisation energies are the quantities necessary to remove the second and third electron from the atom, respectively. Ionisation energy increases when moving from left to right across a period as electrons are bound tightly. When proceeding down a group, the first ionisation energy decreases as an electron in a higher energy level is easier to remove. The trend in the first ionisation energy is the inverse of the atomic radii (see Figure 2.4.3). As atomic radii increase, ionisation energy decreases.
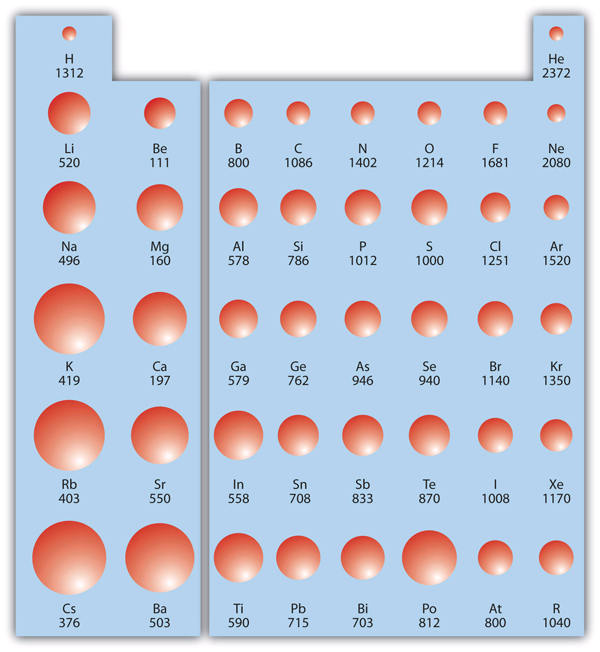
Electronegativity
Electronegativity is the power of an atom in a molecule to attract electrons. The larger the value, the larger the electron-attracting ability. Atoms with higher electronegativity form anions, whereas atoms with smaller electronegativity form cations (see Figure 2.4.4). Electronegativity decreases from top to bottom and increases from the left to the right of the periodic table — similar to the trend of ionisation energy (see Figure 2.4.3). Electronegativity is an important elemental property, as it dictates the types of bonds that can form between elements.
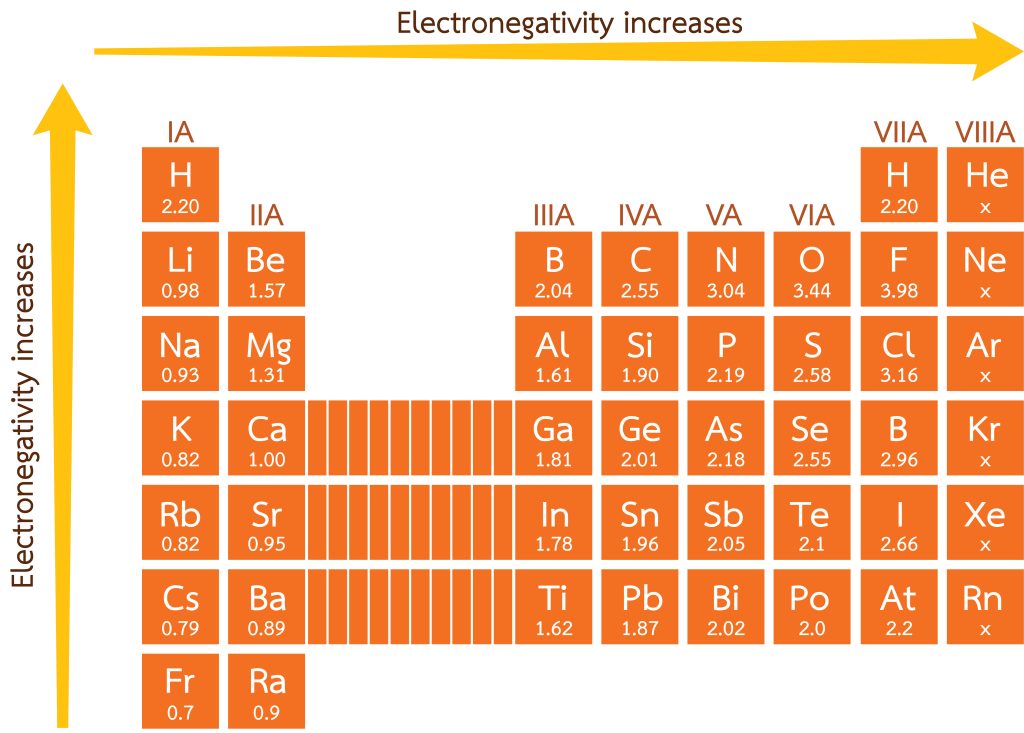
Key Takeaways
- Due to the construction of the periodic table, a number of trends can be seen moving across it.
- Atomic radius of atoms increases from the top to the bottom of a group, and gets larger as one moves from the right to the left.
- Ionisation energy, the energy needed to remove an electron, decreases as atomic radius increases.
- Electronegativity, the power of an atom to attract electrons within molecules, increases alongside ionisation energy
Exercises
Media Attributions
- Periodic table of elements © Torsu - stock.adobe.com is licensed under a All Rights Reserved license
A listing of the shell and subshell labels.
A molecule consisting of only two (di-) atoms.
The amount of energy required to remove an electron.
A negatively charged ion.
A positively charged ion.

