8.6 Heteroatoms and Functional Groups
Learning Objectives
- Identify the different functional groups present in organic molecules.
Functional groups in organic compounds
Functional groups are structural units within organic compounds defined by specific bonding arrangements between specific atoms. Many, but not all, functional groups contain heteroatoms: atoms other than carbon and hydrogen. The structure below (Figure 8.6.1) of capsaicin, the heat-sensation-producing molecule in hot peppers, incorporates several functional groups, labelled in Figure 8.6.1 and explained throughout this section.
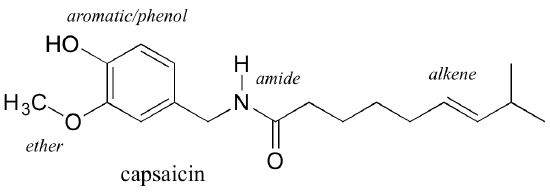
Functional groups are the key structural elements that define how organic molecules act. Our focus for now will be on drawing and recognising each functional group, as depicted by structural formulas and line-bond structures. But as the name implies, functional groups are linked to the behaviour of substances and their impact on the physical and chemical properties of substances. As the structural feature of a wing on an animal is associated with its ability to fly, functional groups on molecules are structural features associated with what those substances can do.
This section includes a quick tour through a collection of functional groups. You are not expected to know all the details entirely after the first read-through. The overview approach can help you appreciate the variety of structures within organic chemistry and can help you begin to build a vocabulary:
- Alkanes: The ‘default’ in organic chemistry (essentially, the lack of any functional groups) is described as an alkane, characterised by single bonds between carbon and carbon or between carbon and hydrogen.
- Alkenes and alkynes: Alkenes (sometimes called olefins) have carbon-carbon double bonds, and alkynes have carbon-carbon triple bonds.
- Aromatic hydrocarbons: The aromatic group is exemplified by benzene and naphthalene.
Further details about hydrocarbons can be found in section 8.1.
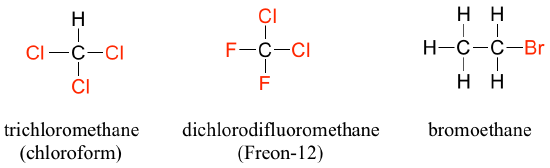
- Alkyl halide: When the carbon of an alkane is bonded to one or more halogens, the resulting compound is called an alkyl halide or haloalkane. For years, chloroform (Figure 8.6.2), a haloalkane with the formula [latex]\ce{CHCl_{3}}[/latex], was a commonly used solvent in the laboratory. This substance was also one of the earlier anesthetic drugs used in surgery. Its use is now highly restricted due to negative health effects, but it remains an important industrial chemical used in the production of PTFE (TeflonTM). Chlorodifluoromethane (see Figure 8.6.2) was used as a refrigerant and in aerosol sprays until the late twentieth century, but its use was discontinued after it was found to have harmful effects on the ozone layer. Bromoethane (see Figure 8.6.2) is a simple alkyl halide often used in organic synthesis. Alkyl halide groups are quite rare in biomolecules.
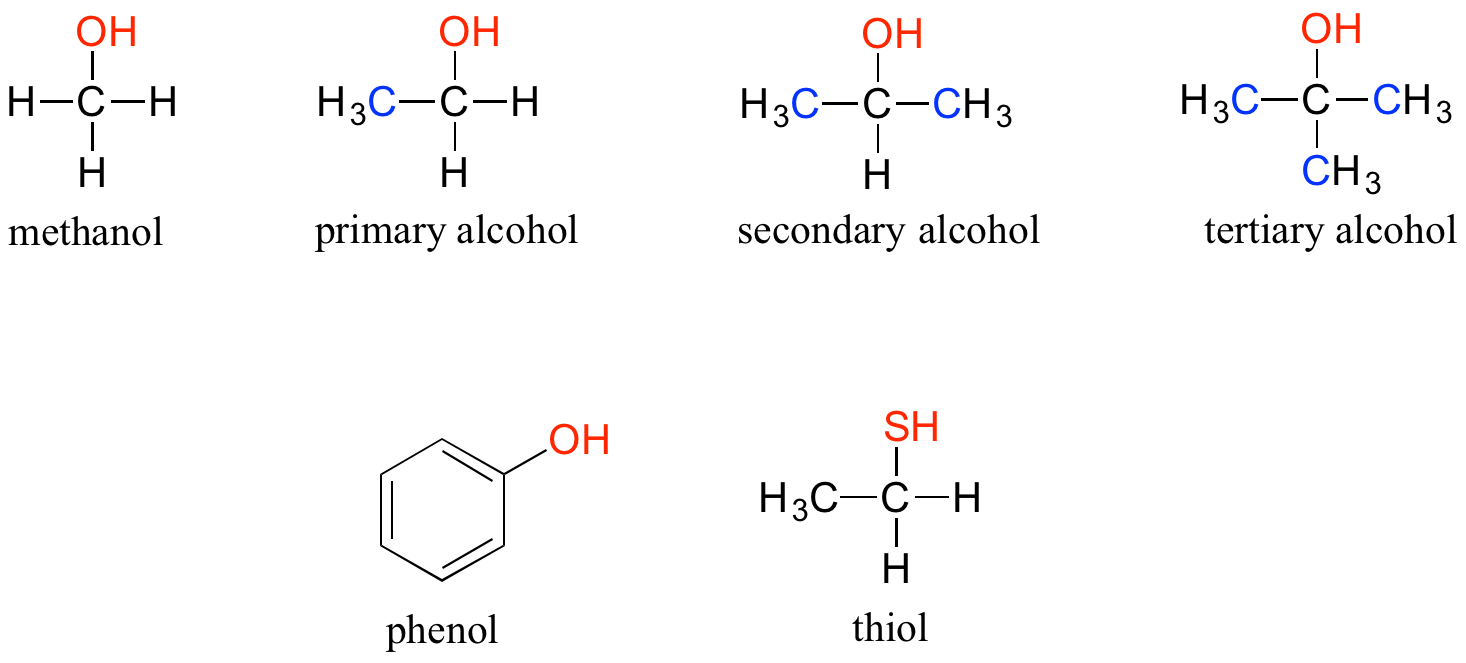
- Alcohol: In the alcohol functional group, a carbon is single-bonded to an [latex]\ce{OH}[/latex] group (the [latex]\ce{OH}[/latex] group, by itself, is referred to as a hydroxyl). Except for methanol, all alcohols can be classified as primary, secondary or tertiary:
- A primary alcohol is an alcohol in which the carbon atom carrying the hydroxyl group is bonded to only one other carbon atom.
- A secondary alcohol is an alcohol in which the carbon atom carrying the hydroxyl group is bonded to two other carbon atoms.
- A tertiary alcohol is an alcohol in which the carbon atom carrying the hydroxyl group is bonded to three other carbon atoms.
When the hydroxyl group is directly attached to an aromatic ring, the resulting group is called a phenol. The sulphur analog of an alcohol is called a thiol (from the Greek thio, for sulphur). The following figure displays the different types of alcohol that we discussed in this section:
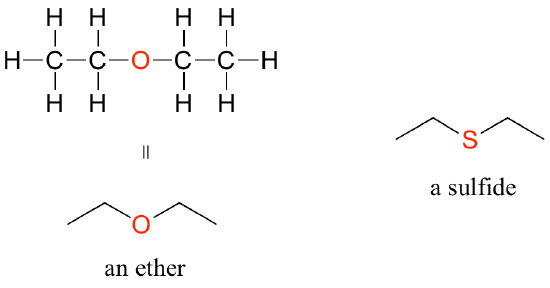
- Ether: In an ether functional group, a central oxygen is bonded to two carbons. Below is the structure of diethyl ether (see Figure 8.6.4), a common laboratory solvent and one of the first compounds to be used as an anaesthetic during operations. The sulphur analog of ether is called a thioether or sulphide.
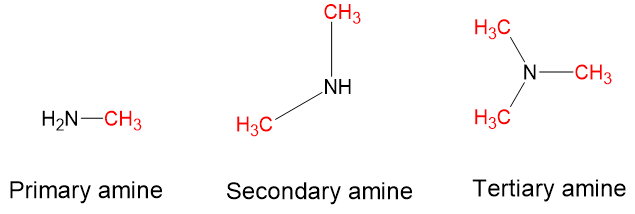
- Amines: Amines are characterised by nitrogen atoms with single bonds to hydrogen and carbon. Just as there are primary, secondary, and tertiary alcohols, there are primary, secondary, and tertiary amines (see Figure 8.6). Ammonia is a special case with no carbon atoms.
- Carbonyl groups: A number of functional groups contain a carbon-oxygen double bond, commonly referred to as a carbonyl. Ketones and aldehydes are two closely related carbonyl-based functional groups that react in very similar ways. In a ketone, the carbon atom of a carbonyl is bonded to two other carbons. In an aldehyde, the carbonyl carbon is bonded on one side to hydrogen and on the other side to carbon. The exception to this definition is formaldehyde, in which the carbonyl carbon has bonds to two hydrogens. Examples of aldehydes and ketones are illustrated in Figure 8.6.6.
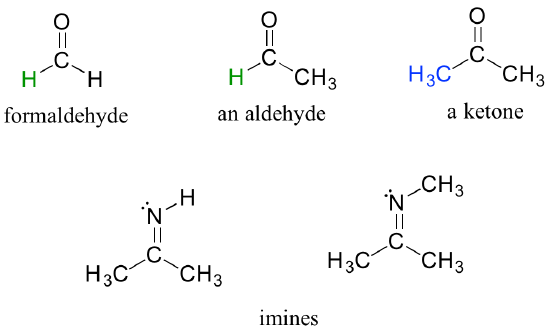
Imines: An imine is a functional group in organic chemistry with a carbon-nitrogen double bond ([latex]\ce{C=N}[/latex]). It is derived from the reaction between a primary amine ([latex]\ce{RNH_{2}}[/latex]) and a carbonyl compound, typically an aldehyde or a ketone. Examples of imines are shown in Figure 8.6.6.
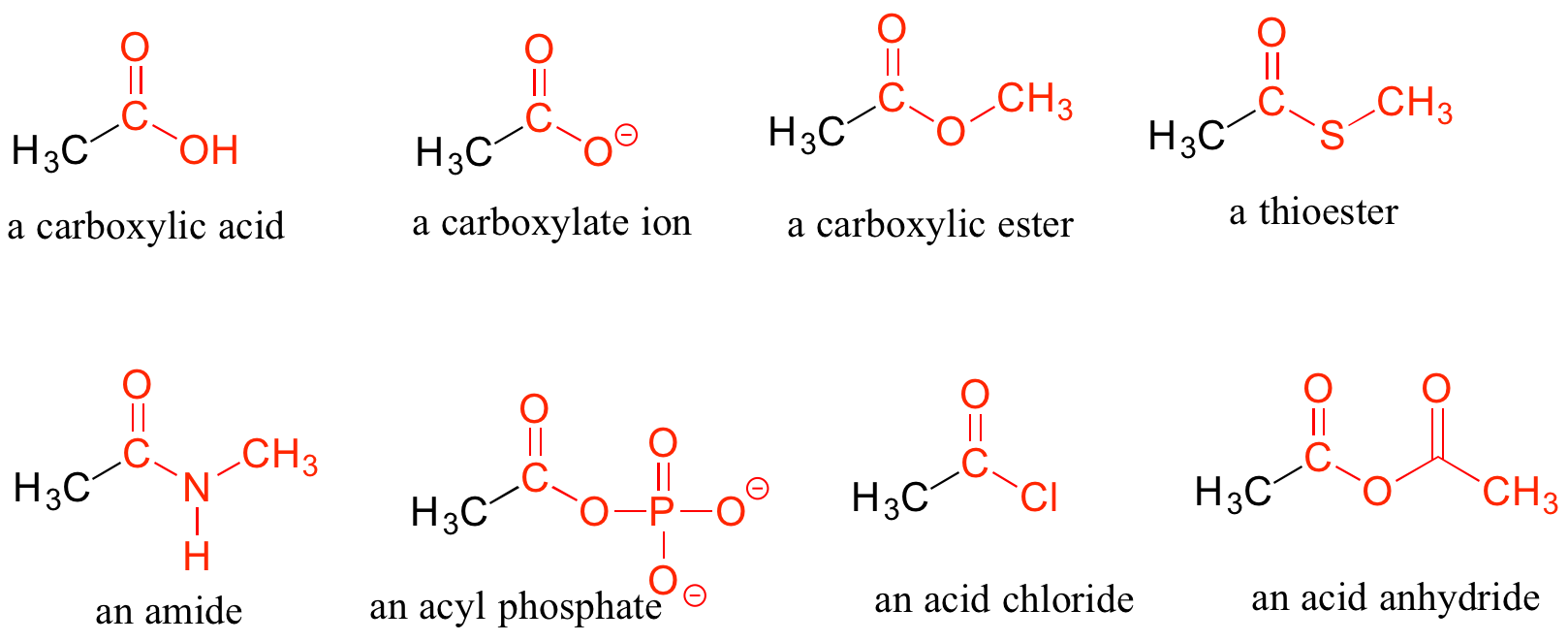
When a carbonyl carbon is bonded on one side to a carbon (or hydrogen) and on the other side to an oxygen, nitrogen, or sulphur, the functional group is considered to be one of the ‘carboxylic acid derivatives’, a designation that describes a set of related functional groups. The eponymous member of this family is the carboxylic acid functional group, in which the carbonyl is bonded to a hydroxyl group. Other derivatives are carboxylic esters (usually just called ‘esters’) and amides. Other carboxylic acid derivatives also exist. Many are common in biology. Examples of carboxylic acids, esters, amides, acid chlorides and acid anhydrides are shown below (see Figure 8.6.7)
Example 8.6.1
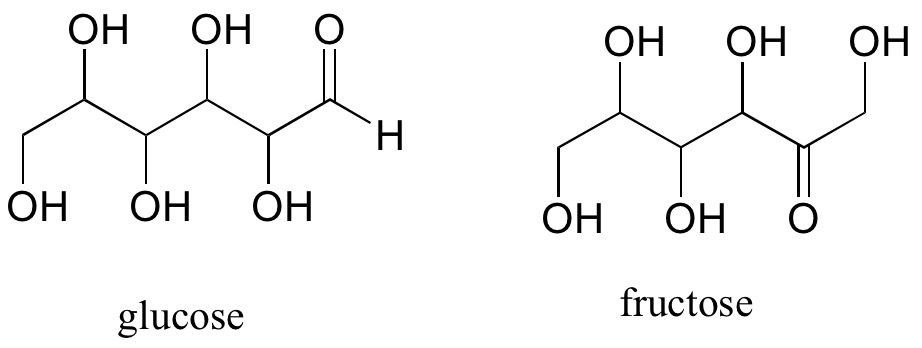
A single compound often contains several functional groups, particularly in biological organic chemistry. The six-carbon sugar molecules glucose and fructose shown below contain aldehyde and ketone groups, respectively, and both contain five alcohol groups.
The hormone testosterone, the amino acid phenylalanine, and the glycolysis metabolite dihydroxyacetone phosphate all contain multiple functional groups, as labelled below.
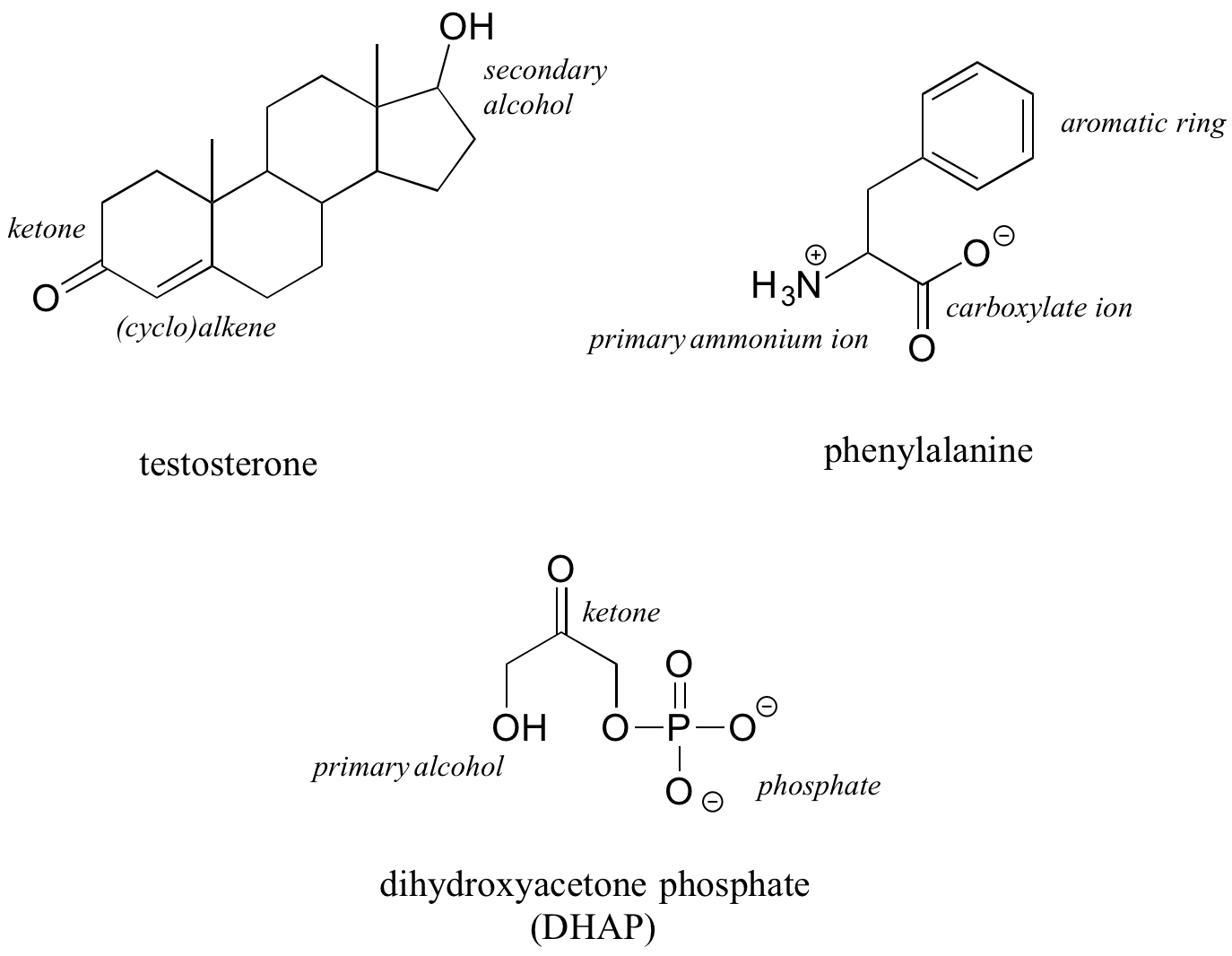
While not a complete list, this section has covered most of the important functional groups we will encounter in biological organic chemistry.
Functional Groups and Organic Nomenclature
As noted earlier, the presence of a functional group frequently appears in the IUPAC name as a suffix. For alkanes, the names end in ‘ane,’ which indicates the absence of any functional group.
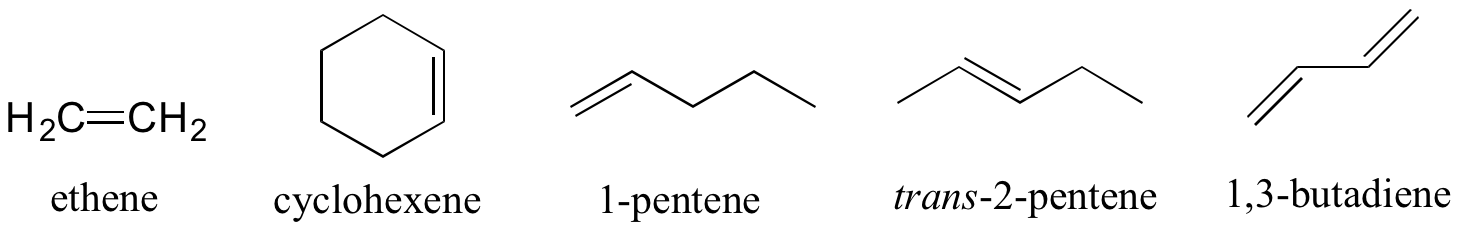
Alkenes are designated with an ‘ene’ ending. Compounds with multiple double bonds are called dienes, trienes, etc, as shown below in Figure 8.6.8.
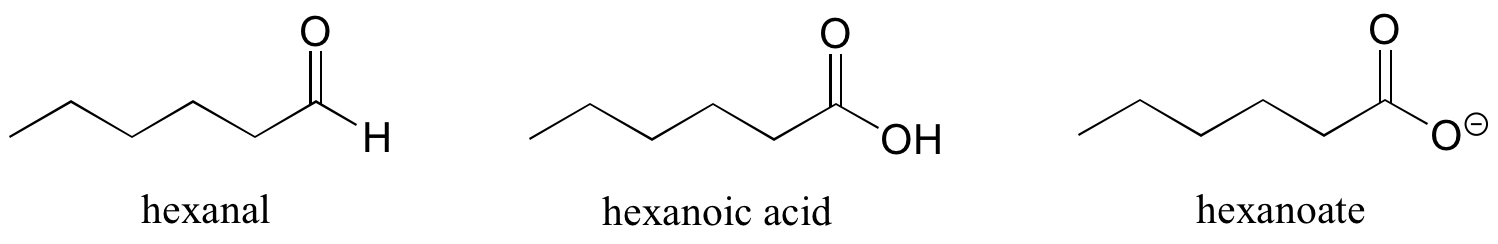
Some groups can only be present on a terminal carbon. Thus, a locating number is not necessary: aldehydes end in ‘al’, carboxylic acids in ‘oic acid’, and their conjugate base carboxylates in ‘oate’, as shown below in Figure 8.6.9.
Other functional groups have their suffixes, as well, and some functional groups affect IUPAC names in more complex ways. Many molecules also have multiple functional groups, complicating the names further.
It is not crucial to learn the details now, but it is valuable to know that the suffix can often be used to identify the presence of a specific functional group on a molecule.
Key Takeaways
- The functional groups in organic molecules determine their reactivity.
- Some functional groups, such as carbonyl groups, amines, and alkyl halides, contain heteroatoms, meaning atoms other than carbon and hydrogen.
- Alkyl halides are alkanes with halogens.
- Alcohols are classified into primary, secondary and tertiary based on the arrangement of carbon atoms around the carbon bonded to the hydroxyl group.
- Carbonyl compounds such as aldehydes, ketones and carboxylic acids contain a carbon oxygen double bond.
- Complex organic molecules can contain more than one functional group.
- The functional group present in an organic molecule contributes to its IUPAC name.
Exercises
Practice Questions

